Rotigotine (Neupro®) Drug Evaluation
Rotigotine (Neupro®) Drug Evaluation
By Caine Hillis, PharmD Candidate, Auburn (AL) UniversityRotigotine (Neupro®) is a new transdermal dopamine agonist.
Mechanisms of Action
- Rotigotine is a non-ergolinic aminotetralin D3/D2/D1 receptor agonist. The precise mechanism is unclear, but it is thought to stimulate D2 receptors within the caudate-putamen in the brain.
- Ropinirole is a non-ergoline dopamine agonist that has a higher specificity to D3 than to D2 and D4 dopamine receptor subtypes.
Indications
- Rotigotine is FDA indicated for early Parkinson's disease.
- Rotigotine (Requip®) is approved for use in the settings of early and advanced PD, as well as restless legs syndrome.
Pharmacokinetics
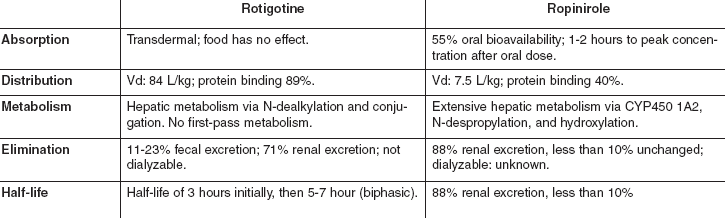
The rotigotine transdermal system avoids the peak-trough effect seen with oral PD medications. Table 1 outlines pharmacokinetic parameters.
Dosing
The rotigotine dose for early PD involves titration. Patients should be started with an initial dose of 2 mg, every 24 hours (single 10 cm2 patch), then increased weekly by 2 mg/24 hours (4 mg/24 hours, 20 cm2 patch), to the highest recommended dose of 6 mg/24 hours (30 cm2 patch).
The rotigotine patch should be applied to clean, dry skin. Serum concentrations of this drug may be higher in patients older than 80 years of age due to skin changes associated with aging. Hydrated, broken, irritated, or warmer skin will also increase permeability. Patients with poor circulation may display decreased serum concentrations.
Ropinirole is dosed the same for both early and advanced PD. Titrate weekly to therapeutic response with an ascending dose schedule: week 1, 0.25 mg TID; week 2, 0.5 mg TID; week 3, 0.75 mg TID; week 4, 1 mg TID; after week 4, may increase daily dosage by 1.5 mg/day on weekly basis up to 9 mg/day, then by up to 3 mg/day weekly to total dose of 24 mg/day; the maximum dose is 24 mg/day.
The ropinirole dose for restless legs syndrome is based on a titrated dose of 0.25 mg for days 1 and 2; 0.5 mg for days 3-7; 1 mg for week 2; 1.5 mg for week 3; 2 mg for week 4; 2.5 mg for week 5; 3 mg for week 6; and 4 mg for week 7.
Contraindications
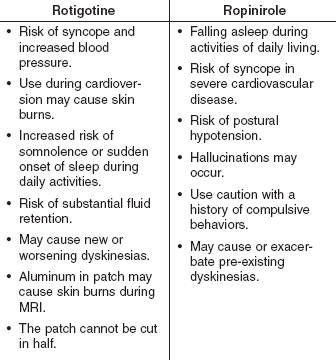
Rotigotine and ropinirole are contraindicated in patients who have hypersensitivity reactions to the drugs or any component of the products. See Table 2 for Warnings and Precautions.
Drug Interactions and Adverse Reactions
Rotigotine's effects may decrease if administered with a dopamine antagonist, such as antipsychotics or metoclopramide. Ropinirole may interact with drugs that are metabolized via CYP450, oral estrogens, and dopamine antagonists.
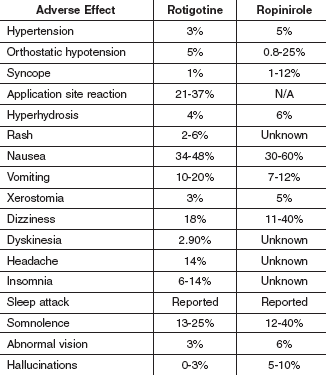
The adverse reactions reported with these two drugs are very similar (see Table 3). One major difference is the application site reaction that only occurs with the rotigotine transdermal system.
Clinical Trial #1: Jankovic J, Watts RL, Martin W, et al. Transdermal rotigotine: Double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol 2007;64:676-682.
Objective: To assess the response to the rotigotine transdermal system in patients with early PD.
Study Design: Randomized, double-blind, multicenter, placebo-controlled study.
Intervention: Patients (n=277) were randomized 2:1 to receive either rotigotine or placebo. Rotigotine doses were titrated to an optimal daily dose to a maximum of 6 mg/day. After titration phase, patients began a 24-week maintenance phase, then were weaned off the drug.
Patient Population
Inclusion Criteria: Male and female aged 30 years or older; diagnosis of idiopathic PD for five years or less; two of the cardinal signs (bradykinesia, resting tremor, rigidity, and postural instability).
Exclusion Criteria: Previous or concurrent therapy with a dopamine agonist or with carbidopa or levodopa within 28 days of baseline; carbidopa or levodopa therapy for more than six months since diagnosis; atypical Parkinsonism; surgical intervention for PD; clinically relevant hepatic, renal, or cardiac dysfunction; a diagnosis of epilepsy, history of adult seizure, stroke, or a transient ischemic attack within the last year; pronounced skin hypersensitivity to adhesive or other transdermal patches or recent unresolved contact dermatitis; known intolerance or hypersensitivity to the antiemetic ondansetron; pregnancy or nursing; or inadequate birth control methods.
Outcomes
Primary Outcome: Percentage of subjects acheiving a 20% response or greater in Unified Parkinson Disease Rating Scale (UPDRS) subtotal from baseline to end of maintenance phase.
Secondary Outcomes: Time for the change from baseline in the UPDRS subtotal score at the end of treatment, prolactin serum concentrations, and QOL measurements.
Results
Primary Endpoint: Patients receiving rotigotine experienced a mean reduction of 15% in UPDRS compared with an increase of 7.3% in patients receiving placebo (P < 0.002).
Secondary Endpoints: The reduction in UPDRS scores remained relatively constant during the first eight weeks of the maintenance phase and gradually diminished with time to the end of the observational safety period. Mean (SD) prolactin serum concentrations decreased from 6.8 (3.54) ng/mL at baseline to 5.0 (5.22) ng/mL by the end of the maintenance phase after treatment with the 6 mg/24 hour rotigotine transdermal system. In the placebo group, mean (SD) prolactin levels were 6.6 (2.83) ng/mL and did not significantly change during the maintenance phase (6.4 [2.59] ng/mL to 7.7 [3.69] ng/mL). The rotigotine group showed slight, statistically nonsignificant improvements from baseline in the EQ-5D QOL Index and the EQ-5D Health State Score. The mean level of QOL (EQ-5D Index) in the rotigotine group at the end of the treatment phase was 0.83 (range, 0.31-1.00; P < 0.05), which indicates a high level of QOL. The placebo group showed deterioration in both QOL measures, with a mean QOL index of 0.77 (range, 0.38-1.00), and in Health State Score, which was statistically significant compared with baseline (P = 0.04).
Strengths
- Randomized, double-blind trial
- Intention-to-treat analysis
- Appropriate patient demographics (primarily white males, average age 63 years)
- Appropriate inclusion criteria
- FDA-approved dosage employed
Limitations
- Extensive exclusion criteria
- Only conducted in United States and Canada
- Small patient population
- Length of study
Author's Conclusions
- Rotigotine consistently demonstrated statistically significant and clinically relevant efficacy over placebo in patients with early PD.
- As a once-daily medication for PD, rotigotine provides the basis for continuous dopaminergic stimulation for at least six months.
- Rotigotine treatment is well tolerated and favorably affected QOL in patients with early PD.
Clinical Trial #2: LeWitt PA, Lyons KE, Pahwa R, et al. Advance Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 2007;68:1262-1267.
Objective: To assess the efficacy and safety of rotigotine in advanced PD subjects with > 2.5 hours of daily "off " time.
Study Design: A randomized, double-blind, placebo-controlled trial.
Intervention: Subjects were randomized to receive placebo patches (n = 120) or rotigotine up to either 8 mg/24 hours (n = 120) or 12 mg/24 hours (n = 111) for a 24-week maintenance phase.
Patient Population
Inclusion criteria: Subjects (>30 years of age) had the diagnosis of idiopathic PD for at least three years, with clinical features of bradykinesia plus at least one additional cardinal feature. All subjects had a Hoehn and Yahr Stage between II and IV in both the "on" and "off " states and were not demented (Mini-Mental State Examination score > 25). Study participants were receiving at least 200 mg/day of levodopa administered in two or more daily doses and in a regimen stable for at least 28 days prior to baseline. All subjects had inadequate relief of Parkinsonism as judged by the treating investigator.
Exclusion criteria: DA, catechol-O-methyltransferase inhibitor, methylphenidate, amphetamines, monoamine oxidase type A inhibitors, reserpine, alpha-methyldopa, or neuroleptics including clozapine and quetiapine were not permitted within 28 days of baseline. Patients with prior pallidotomy, thalamotomy, deep brain stimulation, or tissue transplant to the brain also were excluded from participation.
Outcomes
Primary Outcomes: Change in the absolute time spent "off " and the percentage of subjects achieving >30% response ("responders") in absolute time spent "off " from baseline to the end of the maintenance phase.
Secondary Outcomes: Change from baseline to the end of the maintenance phase in time "on," "on without troublesome dyskinesia," and "on with troublesome dyskinesia" states; the number of "off " periods; and "on"/"off " status of the subject after waking.
Change from baseline to the end of the maintenance phase in UPDRS activities of daily living and motor sections in the "on" state, and complications of therapy (Part IV) were assessed at each visit.
Results
Compared to placebo, there were significant decreases in mean "off " time of 1.8 hours/day for the rotigotine 8 mg/24 hours group and 1.2 hours/day for the 12 mg/24 hours group. For the rotigotine 8 mg/24 hours and 12 mg/24 hours groups, >30% responder rates were 56.6% and 55.1%, respectively, compared to the 34.5% placebo response. "On" time without dyskinesia after awakening was more than double in both rotigotine treatment groups vs. placebo.
Drug-related adverse effects included typical dopaminergic side effects, which were generally mild/moderate in intensity. Patch application site reactions were mild to moderate and transient in the majority of instances.
Strengths
- Randomized
- Double-blind trial with three treatment arms
- Intent-to-treat analysis
Limitations
- Only 260 patients completed the study
- Relatively small patient population
- Limited to United States and Canada
Author's Conclusion
The addition of rotigotine at both 8 mg/24 hours and 12 mg/24 hours significantly reduced "off " time for subjects with advanced PD not optimally controlled with levodopa. The 24-hour transdermal effect offered the potential for antiparkinsonian control extending through the night into the morning hours after awakening.
Cost
At Huntsville Hospital, the rotigotine patch costs: $2.25 (2 mg) and $7.70 (4 mg and 6 mg); a 9 mg oral regimen of ropinirole costs $6.12 and the maximum recommended dose of 24 mg costs $18.64.
Recommendation
Transdermal rotigotine is recommended for formulary status for use in certain situations, for example when medications can no longer be taken by mouth. Rotigotine might be used as an adjunct to levodopa in a PD patient whose disease has progressed to the point where he or she can no longer swallow medications. Rotigotine can be applied at any time and has the potential to increase compliance over oral treatments that are taken multiple times per day. Rotigotine use may also to avoid the GI problems associated with oral dopamine agonists. By achieving steadier serum concentrations and avoiding the peak/trough effect, patients taking this drug experience less "off" time compared to oral PD agents, which improves their quality of life.
Rotigotine (Neupro®) is a new transdermal dopamine agonist.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
