Common Arrhythmias for the Primary Care Clinician
Common Arrhythmias for the Primary Care Clinician
Authors: Brian Olshansky, MD, Professor of Medicine, Director, Cardiac Electrophysiology, University of Iowa Hospitals, Iowa City, IA; Benjamin Rhee, MD, Electrophysiology Fellow, University of Washington Medical Center, Seattle; and Joel Wilson, MD, Internal Medicine Resident, University of Washington Medical Center, Seattle.
Peer Reviewer: Saleem Ahmad, MD, Electrophysiologist, PriMed Cardiology, Kettering, OH.
Managing cardiac arrhythmias is a unique and complex challenge in the primary care setting. The clinician must balance proper initial assessment, long-term management schemes and effective acute and chronic treatment approaches with appropriate triage to a cardiac specialist and/or an electrophysiologist. The treating clinician must be able to diagnose the arrhythmia (if possible), understand the risks to the patients, and plan an acceptable therapeutic strategy. Available treatment options are evolving rapidly.
Evaluation of the patient begins with a careful history and physical examination. Testing should progress from the simplest, most cost-effective, and safest tests to those with higher levels of risk, invasiveness, and cost as necessary for a definitive diagnosis. Occasionally, a physician may proceed directly to a high-risk, expensive procedure, such as an electrophysiological study, before obtaining a 24-hour electrocardiographic recording.1
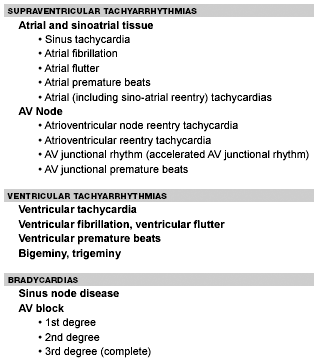
The three main reasons to evaluate and treat arrhythmias are to eliminate symptoms, prevent death, and reduce long-term risks associated with specific arrhythmias and/or clinical conditions. Common arrhythmias are divided into tachycardias (rate greater than 100 beats per minute [bpm]), bradycardias, and ectopy. (See Table 1.)
| Table 1. Cardiac Arrhythmias |
|
|
Tachyarrhythmias are divided into narrow and wide QRS complex. Narrow complex tachycardia refers to a rhythm with a QRS duration less than 120 msec and rates greater than 100 bpm. They usually are supraventricular tachycardias. They further are divided into those that require only atrial tissue for initiation and maintenance (i.e., atrial flutter, atrial fibrillation, and atrial tachycardias) and those that require the AV node (i.e., AV nodal reentry, junctional tachycardia, and AV reentry utilizing a bypass tract). Wide complex tachycardias have a QRS width of 120 ms or greater. They include ventricular tachycardia and supraventricular tachycardias with aberrant conduction or pre-excitation.
Bradyarrhythmias are rhythm disturbances with rates less than 60 bpm. Sinus bradycardia is the most common bradycardia. It occurs commonly in children and adults, with rates as low as 30 bpm during sleep. Sinus bradycardia generally is not associated with adverse clinical outcomes. However, sinus bradycardia may be associated with symptoms and/or pathophysiological conditions that may cause serious problems.
Cardiac Arrest
Sudden cardiac death is an unexpected natural death due to cardiac causes, heralded by abrupt loss of consciousness within 1 hour of symptom onset.1 The definition is misleading in that not all affected individuals actually die, and those who survive cardiac arrest are said to have aborted sudden cardiac death. Nevertheless, approximately 300,000 deaths are estimated to occur annually, making this one of the major causes of death in the United States.2 Ventricular fibrillation is the most common cause of sudden cardiac death. Early defibrillation and CPR can abort many of these deaths but, nevertheless, this rarely occurs in most major metropolitan centers.
Management of survivors of cardiac arrest is complex. Provoking factors, such as underlying heart disease, ischemia, myocardial infarction, valvular disease, infiltrative cardiomyopathy, dilated cardiomyopathy, medications (including antiarrhythmic agents and diuretics), electrolyte imbalances, and other agents, must be considered. Clinical conditions such as acidosis, hypoxia, hypovolemia, hypothermia, and hypomagnesemia are associated with cardiac arrest. Unfortunately, in the majority of cases, no specific precipitating factor can be identified other than the presence of underlying heart disease. Sudden cardiac death often is the initial clinical event. Survivors of sudden cardiac death should be considered for an implantable cardioverter-defibrillator (ICD), in the absence of a reversible cause of their arrest, as long as they otherwise are likely to survive.
Evaluating Symptoms Caused by Arrhythmias
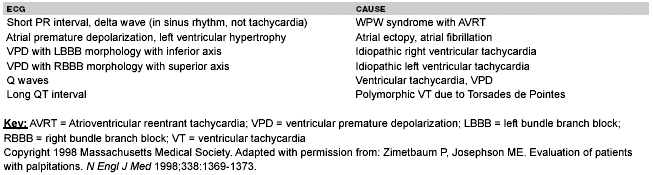
The presence of palpitations is one of the most common problems experienced by outpatients who present to primary care physicians and cardiologists. (See Table 2.) Palpitations have been variably described as flip-flopping in the chest, rapid fluttering in the chest, and pounding in the neck. They may be sustained or intermittent, regular or irregular. Frequently, the circumstances during which palpitations occur can help identify their cause. Palpitations may be associated with panic or anxiety. One study of palpitations showed that panic disorder was the cause in 20% of patients.3 There is good evidence that psychiatric disorders are a common cause of palpitations. However, palpitations often are considered panic disorders when in fact they are due to an arrhythmia, so this diagnosis should not be accepted until true arrhythmic causes have been excluded.
| Table 2. Palpitations ECG Clues |
|
|
Other symptoms also can suggest the presence of arrhythmia. Syncope can be caused even by brief runs of nonsustained ventricular tachycardia and by supraventricular tachycardia, and may or may not be associated with palpitations. Cardiac rhythm disturbances may manifest as chest or neck discomfort, dyspnea, weakness, and anxiety.
In evaluating symptoms presumed to be related to the arrhythmia, the detailed history must focus on symptoms related to the arrhythmia, including its timing and triggers. Palpitations associated with dizziness, or syncope may suggest the presence of tachycardia.
Underlying illnesses may contribute to symptoms. Secondary consequences of arrhythmias, often due to underlying heart diseases such as heart failure, tachycardia mediated cardiomyopathy, and thromboembolic disease, can cause symptoms and should be investigated carefully. Fluctuations in sympathetic and vagal tone, hormonal changes, elevation of venous pressure, and reduced cardiac output also may alter or even provoke arrhythmias. Dietary habits, especially those regarding intake of tea, caffeine, and alcohol, must be considered. Supraventricular tachycardias may be exacerbated during menses, possibly related to either plasma levels of ovarian hormones or electrolyte shifts.5 They also may exhibit circadian patterns, which worsen during certain times of the day or night.6,7 The relationship to exercise should be investigated as well. Clues may be derived from the onset and termination of the symptoms, in addition the length of time the patient has experienced symptoms.
Following the interview and physical examination, a 12-lead electrocardiogram (ECG) should be obtained. An ECG and/or a rhythm strip alone recorded during the arrhythmia may be enough to determine the type of arrhythmia and whether the patient's symptoms are related to the arrhythmia. The PR interval, QT interval, the QRS width, the PR relationships, the rate, and the rhythm should be considered. If present, delta waves, suggesting Wolff-Parkinson-White syndrome, evidence for ventricular hypertrophy, or indications of myocardial infarction will help direct further evaluation.
Ambulatory Monitoring
Ambulatory monitoring will help make the diagnosis as well. Continuous loop recorders may be more cost-effective and efficacious than ambulatory (Holter) monitors for the evaluation of palpitations, depending on the frequency of symptoms.4 However, if the palpitations are sustained or are tolerated poorly, an electrophysiologic study, with or without prior ambulatory monitoring, may diagnose the cause. Ambulatory monitoring is the test of choice for daily symptoms suggestive of arrhythmias. (See Table 3.)
| Table 3. Indications for Ambulatory ECG Monitoring |
|
|
In patients with less frequent symptoms, an event monitor or loop recorder, a device that can be provided to the patient for up to one month, is a better choice with which to make the diagnosis. Transtelephonic ECG devices exist as either continuous or non-continuous formats. These ECG signals are received at a base station with a demodulator and an ECG strip chart recorder. A memory exists for short periods of time when the patient may not have telephone access. Lastly, implantable monitors ("Reveal" monitor) are now available that can be implanted subcutaneously for periods of up to 15 months.8
Once an arrhythmia is diagnosed, several factors will determine whether to admit a patient or to initiate treatment on an outpatient basis. This includes the type of the arrhythmia, the severity of associated symptoms, and the condition of the patient. The following section will discuss the implications of specific arrhythmias.
Sinus Tachycardia
The maximal heart rate may be as high as 200 bpm in young people, and 150 bpm in older individuals. Sinus tachycardia frequently reflects underlying processes, such as metabolic state, fever, medications, hypovolemia, shock, anxiety, pulmonary embolus, and many other causes. Sinus tachycardia can have significant clinical consequences, especially in people with underlying heart disease. Patients with coronary artery disease at risk for ischemia or valvular disease with an obstructive lesion may not tolerate this arrhythmia well. Therapy should be directed at correction of the underlying problem whenever possible.
Inappropriate sinus tachycardia occurs when affected individuals have chronic tachycardia without heart disease or other reasons for tachycardia. Individuals have an abnormally high resting heart rate that is accompanied by symptoms of fatigue and palpitations. Their heart rate increases rapidly with minimal exercise, and they may experience exercise intolerance. Unfortunately, the clinical presentation can be quite variable, and the degree of impairment varies from asymptomatic individuals to patients who are incapacitated by their symptoms. Although there are only small randomized controlled trials, this disorder, most commonly in young women, often is treated successfully with beta-blockers and calcium channel blockers to control heart rate. Sinus node modification or ablation may be necessary in extreme cases, especially in patients without autonomic dysfunction.9,10
Postural Orthostatic Tachycardia Syndrome (POTS) must be considered before a diagnosis of inappropriate sinus tachycardia can be made. This condition commonly is seen in young patients with symptoms such as fatigue, lightheadedness, and exercise intolerance when in the upright position. These symptoms are associated with tachycardia without a fall in blood pressure. Symptomatology is due to an abnormal distribution of central sympathetic tone to the heart and vasculature, resulting in an overall enhancement of noradrenergic tone at rest and by a blunted postganglionic sympathetic response to standing.11 Diagnosis is made by history and tilt-table testing. During tilt-table testing, symptoms usually are reproducible and an increase in heart rate of 30 bpm or greater is seen, typically early during the examination.12 Therapies for this illness may include fludrocortisone for those patients with low circulating volume. Phenylephrine, the alpha-1 agonist, has been used to improve symptoms and hemodynamics during tilt-table testing.13 Although the sinus rate can be slowed by sinus node modification, clinical symptoms did not improve significantly in a small clinical trial.14
Atrial Fibrillation
Atrial fibrillation (AF) is the most common tachycardia requiring therapy. AF occurs in approximately 1% of the general population. Its prevalence increases with age, affecting up to 10% of the population older than 80 years.15 Presentation can range from asymptomatic to hemodynamic collapse. Commonly, symptoms of fatigue and dyspnea occur. Less commonly are symptoms of chest pain, pulmonary edema, or syncope. AF also may be noted in patients presenting with stroke and can be the cause. AF frequently is associated with hypertension,16 rheumatic heart disease,17 valvular heart disease, heart failure,18 and coronary artery disease. It also has been associated with hyperthyroidism,19 caffeine20 and alcohol intake,21 infections, chronic obstructive pulmonary disease (COPD), and pulmonary embolism.22 Surgery, especially cardiac surgery, also is associated with a 35-50% risk of AF in the post-operative period.23
Lone AF is a clinical condition occurring in patients usually younger than 60 years who have no clinical evidence of cardiovascular disease and are at low risk for thromboembolism. Discussion continues as to whether diabetes should be considered a condition that alters the risk for developing thromboembolism in these patients. Large clinical AF trials largely excluded patients with lone AF. A study from the Mayo Clinic, in which 2.7% of AF patients had lone AF, found a 15-year likelihood of embolic stroke to be 1.3%, about the same as risk of bleeding from warfarin.24
In patients with lone AF or low-risk AF, aspirin may be a viable alternative to warfarin.25 While there is no consensus as to what constitutes low-risk AF regarding stroke, the CHADS2 (congestive heart failure, hypertension, age, diabetes and stroke as twice the risk of the others) score generally is used.26-30
Age older than 65 years (or 75 years in some reports), diabetes, hypertension, congestive heart failure, and prior stroke or transient ischemic attack all increase the risk of stroke in patients with AF. The vast majority of patients with AF should be anticoagulated with warfarin with a goal of international normalized ratio (INR) of 2.0-3.0. This has been demonstrated in several large randomized controlled clinical trials. These trials have consistently demonstrated a significant reduction in stroke risk in patients treated with therapeutic levels of warfarin vs. aspirin or placebo.31,32
Patients with a history of AF still may have increased risk of stroke even after resumption of sinus rhythm, especially if they have other risk factors for stroke. The authors recommend continuing anticoagulation with warfarin in patients with AF unless a contraindication exists.
Recent trials such as AFFIRM,33 RACE,34 and PIAF35 compared rate control vs. rhythm control strategies in patients at risk for stroke. Most were symptomatic. Conventional wisdom in the past had recommended trying to return patients to sinus rhythm with the use of antiarrhythmic drugs and cardioversion, if needed. However the latest data suggest that rate control is not associated with worse survival than rhythm control.33 The AFFIRM and RACE trials indicate that rhythm control is no longer to be considered superior to rate control as a strategy to treat AF.33,34
Rhythm control, if used, may be abandoned early if not fully satisfactory to maintain sinus rhythm. While some patients may benefit symptomatically from sinus rhythm, there is no mortality advantage and no reduction in risk of stroke with this approach. In patients for whom sinus rhythm is important to maintain, cardioversion should be considered as an adjunct to medical therapy with antiarrhythmic drug therapy. To optimize chances of successful long-term cardioversion, steady-state antiarrhythmic therapy should be considered before DC cardioversion for patients with recurrent or refractory AF.36 Finally, in refractory symptomatic patients, referral to an electrophysiologist for further treatment is appropriate. Symptom management may include attempts to maintain sinus rhythm with repeat cardioversions with other antiarrhythmic drugs, such as flecainide, propafenone, or dofetilide, but for patients who remain symptomatic or become intolerant, ablation is an option. New endocardial catheter and epicardial ablation procedures for AF are promising and may relieve symptoms in as many as 60-80% of patients, particularly for patients with paroxysmal or persistent AF. For patients who remain symptomatic after other treatment modalities have been exhausted, ablation of the AV node with a permanent pacemaker may be considered.37
Atrial Flutter
Atrial flutter is a common atrial arrhythmia that frequently is seen in association with AF. It is characterized by a regular atrial rhythm of 250-450 bpm, often approximately 300 bpm. The ventricular rhythm varies, but classically is 150 bpm, representing two atrial depolarizations for every beat conducted into the ventricle (termed 2:1 conduction) through the AV node. The atrial electrical circuit is circular, giving rise to flutter waves in a "sawtooth" pattern. When the impulses contain the isthmus of tissue between the inferior vena cava and the tricuspid annulus as a critical part of the circuit, it is termed typical, or isthmus dependent, atrial flutter. Clinically, atrial flutter may be associated with a rapid ventricular response and may cause symptoms of lightheadedness, palpitations, or dyspnea similar to AF.
If pharmacologic cardioversion is desired, ibutilide may be considered, but it rarely is used. (See Table 4.) It also may help facilitate DC cardioversion if the patient remains resistant to shock alone. Success rate of chemical cardioversion of atrial flutter with ibutilide approaches 60%.38 Clinicians using ibutilide should monitor the patient for the development of torsades de pointes for at least 4 hours after the dose. Prior to use of ibutilide, review of patient electrolytes should be performed, with attention to potassium and magnesium. Medications should be reviewed and agents that can prolong QT intervals should be discontinued if possible. Administration of ibutilide should be performed in a closely monitored setting with an external defibrillator at the bedside.
| Table 4. Pharmacological Therapy of Atrial Flutter |
|
|
Direct current cardioversion may be used for acutely hemodynamically compromised patients and is highly successful at converting flutter to sinus rhythm. Rapid atrial pacing can terminate this arrhythmia, and should be considered for those patients who have epicardial atrial pacing wires in place after open-heart surgery.
Radiofrequency ablation can be curative in these patients, with an efficacy reported as high as 95% for long-term elimination of typical atrial flutter.39 Radiofrequency ablation is associated with a low risk of complications, and therefore should be considered as first line therapy for patients.40 The success rate of ablation is lower in atypical atrial flutter, which includes non-isthmus dependent flutter, scar related atrial flutter, and left atrial flutters.
No prospective randomized controlled trials provide information about the relative risk of thromboembolic complications in patients with atrial flutter but retrospective data is available concerning stroke risk. The authors favor warfarin anticoagulation for episodic atrial flutter. If the patient undergoes radiofrequency ablation, and sinus rhythm persists for at least four weeks following the procedure, then warfarin may be discontinued, assuming there are no other indications for anticoagulation.
Atrial Tachycardias
Other atrial tachycardias typically arise from ectopic atrial foci and usually have rates that range between 150 and 250 bpm. The P waves may be shaped abnormally depending on the location of the ectopic atrial foci. In addition to tachycardias caused by atrial premature beats, there are four other common atrial tachycardias.
Atrial tachycardia, which is more common in elderly patients, usually starts and stops abruptly. Sinoatrial reentry tachycardia can be considered a form of atrial tachycardia. It can be associated with AV block.41 Ectopic atrial tachycardia, a chronic arrhythmia that may appear in children and young adults, can be difficult to distinguish from sinus tachycardia. This form of tachycardia may be associated with tachycardia-induced cardiomyopathy due to persistent tachycardia.
Another atrial tachycardia is multifocal atrial tachycardia. It is characterized by at least three different morphology P waves and varying PR intervals. The ventricular rhythm on examination may be irregular. It is distinguished from AF by the presence of P waves. This arrhythmia often is seen in association with digoxin toxicity, chronic pulmonary disease, or hypoxia.
If atrial arrhythmias are symptomatic or antiarrhythmic drugs do not work, ablation should be considered. Recently a great deal of attention has been devoted to ablation of atrial tachycardias. Ablation of these focal atrial tachycardias is dependent on the ability to map them successfully. There is a high success rate when experienced operators perform these ablations.
Atrioventricular Nodal Reentry Tachycardia
Another common supraventricular tachyarrhythmia is AV nodal reentry, which accounts for 60-65% of supraventricular tachycardia cases.40 Patients may present with palpitations, dizziness, or neck pulsations. Females have a higher prevalence than males. Rates of 140-250 bpm are common in this type of supraventricular tachycardia.
AVNRT can be typical or atypical. Typical AVNRT, termed slow-fast AVNRT (antegrade conduction down a slow pathway), does not have P waves visible on the surface ECG because the atria and ventricles are depolarized simultaneously when conduction occurs down the slow pathway and retrograde up the fast pathway. Atypical AVNRT, also known as fast-slow AVNRT, may have P waves seen following the QRS complex, rather than inscribed in the QRS complex as in typical AVNRT. In this case, the P waves are inverted in the inferior leads reflecting activation from the AV node. Either form may be treated with radiofrequency ablation with a high degree of success (> 95%),42 low rate of recurrence, and low rate of complications (< 2%).43
Some patients may elect not to undergo or may be unable to undergo the ablation procedure. In these patients, beta-blockers, calcium channel blockers, and digoxin may be used to treat the arrhythmia. If these fail, and the patients do not have structural heart disease, flecainide and propafenone (Class Ic drugs) have been used with some success. These therapeutic choices are less frequent due to the high success rate of catheter ablation in treating these arrhythmias.
Atrioventricular Reciprocating Tachycardias
Atrioventricular reciprocating tachycardias are seen when extra nodal ("accessory") pathways connecting the atria and the ventricles are present. However, pathways may conduct antegrade, retrograde, or bidirectionally. Accessory pathways that conduct antegrade may cause delta waves to appear on the surface ECG. The degree of pre-excitation seen on ECG depends on the relative contribution of the atrioventricular (AV) node vs. the accessory pathway. Wolff-Parkinson-White syndrome is diagnosed in patients who have both an accessory pathway on the electrocardiogram (a delta wave and a short PR interval) and symptoms of tachycardia. Catheter ablation is recommended as first-line therapy for symptomatic (and even asymptomatic patients) patients with Wolff-Parkinson-White syndrome. Ablation is the preferred option for patients with AF and Wolff-Parkinson-White pattern on the ECG.
Hospitalization
An important decision involves the setting for arrhythmia evaluation. Hospital management should be considered strongly if the patient has significant underlying heart disease such as cardiomyopathy with heart failure, or coronary heart disease with active ischemia. Highly symptomatic or patients with uncontrolled arrhythmias also should be considered for inpatient treatment. Admission is required for patients with life-threatening arrhythmias that require rapid conversion such as polymorphic ventricular tachycardia (VT), prolonged QT interval in patients with syncope, or other rapid tachyarrhythmias. Additionally, antiarrhythmic drugs paradoxically can precipitate arrhythmias, a phenomenon known as proarrhythmia. Admission to monitor for the development of proarrhythmia should be considered strongly during antiarrhythmic drug initiation. QRS width and QT interval should be carefully examined, as well as electrolytes and renal and liver function.
Proarrhythmia
Torsades de pointes, monomorphic ventricular tachycardia, sinus bradycardias, AV block, and cardiac arrest are all potential drug-related proarrhythmic responses.
Torsades de pointes is one proarrhythmic effect of a medication that lengthens the QRT interval. It is characterized by a ventricular tachycardia with a twisting of the peaks of the QRS complexes across an imaginary isoelectric line. Bradycardia often is present, the QT interval is prolonged, and the arrhythmia most often is induced after a short-long-short cycle of RR intervals. First recognized as a complication of quinidine, identification of proarrhythmia has resulted in the more selective use of antiarrhythmic drugs. Quinidine use has decreased, especially among cardiologists and electrophysiologists, replaced by amiodarone and sotalol. These drugs and dofetilide can cause torsades de pointes as well. For non-antiarrhythmic drugs associated with torsades, please refer to www.torsades.org.
The exact risk of proarrhythmia is not certain. It is estimated to range from 1-5% with various antiarrhythmic drugs. The risk varies with the type of arrhythmia, presence of structural heart disease, QT interval, preexisting conduction disturbances, age, presence of heart failure, electrolyte disturbances, and left and right ventricular function.45 In fact, the risk of proarrhythmia is higher for class IC drugs than for class 1A drugs in patients with structural heart disease.46 Unfortunately proarrhythmia is not usually predictable. Some medications, such as sotalol, the class IC drugs, and N-acetyl procainamide (metabolite of procainamide), may have increased risk of proarrhythmia at higher doses.
Ventricular Arrhythmias
Ventricular premature beats (VPBs) are common and increase in prevalence with age and in the presence of heart disease.47 They are a marker for sudden death, especially in patients with structural heart disease. As a result of this observation, trials were undertaken to determine whether suppression of the VPBs would improve survival. The Cardiac Arrhythmia Suppression Trial (CAST) enrolled patients with six or more VPBs per hour following myocardial infarction, and randomized these patients to encainide, flecainide, or placebo. The trial was terminated prematurely when an increase in mortality was found in patients treated with antiarrhythmic drugs (drug proarrhythmia) compared to placebo.46 Although the increased mortality was not definitively demonstrated to be due to the antiarrhythmic agents, that is the prevailing belief.
The role of amiodarone to suppress VPBs was assessed in the CAMIAT trial. The EMIAT trial was designed to study amiodarone as prophylaxis against arrhythmic death. Both were placebo-controlled trials conducted in patients with recent myocardial infarctions. Although these trials had different designs, both failed to show a significant decrease in overall or cardiac mortality.48-50 Based on these trials, use of antiarrhythmic agents for suppressing VPBs has been limited to symptomatic patients. Amiodarone has become the preferred drug for symptomatic arrhythmias after myocardial infarction. There is no present role for prophylactic amiodarone for any patients.
Ventricular Tachycardia
Ventricular tachycardia is the most common wide complex tachycardia.51,52 However, distinguishing this rhythm from other wide complex tachycardias can be difficult, and misdiagnosis may be common.53 A number of diagnostic algorithms have been suggested, such as the Brugada criteria,54 but these have limited utility in clinical practice because of their generally low specificity.55,56 Given the potential serious sequelae of misdiagnosis, wide complex tachycardias should be presumed to be ventricular tachycardia until proven otherwise.
Ventricular tachycardia is a tachycardia (greater than 100 bpm) of three or more consecutive ventricular beats. Clinical and electrocardiographic clues may assist in the diagnosis. Age greater than 35 years, or prior history of coronary artery disease or congestive heart failure favor ventricular tachycardia over supraventricular tachycardia.51,57 Cannon A waves and electrocardiographic evidence of AV dissociation argue for ventricular tachycardia. Other useful ECG findings arguing in favor of ventricular tachycardia include presence of fusion or capture beats, an extreme "northwest" axis, QRS concordance in precordial leads, and specific morphology criteria (variations in RBBB and LBBB patterns, polymorphic vs monomorphic waveforms).58 Whenever strips or ECGs are available for review, they clearly should be included in the patient's chart.
ICD Therapy
Several multicenter, randomized, controlled clinical trials have demonstrated impressive survival benefits for the prophylactic implantation of ICDs in both ischemic and nonischemic populations. The MADIT-II trial, showed that in patients with history of myocardial infarction and ejection fraction less than 30%, ICD therapy conferred a striking survival benefit compared to "best medical" drug therapy.59 The Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial showed that patients with nonischemic cardiomyopathy and ejection fraction less than or equal to 35% who received ICD therapy trended toward reduction in the relative risk of death as compared with those who received medical therapy.60 The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), the results of which were announced earlier this year, showed that in a group of 2,521 patients with moderate heart failure with ejection fraction less than or equal to 35%, half of whom had nonischemic cardiomyopathy, the use of ICDs reduced the rate of death by 23% over a period of five years as compared with standard medical therapy with or without amiodarone.61 The Centers for Medicare and Medicaid Services (CMS) estimates that expanding the indications for implanting ICDs to include nonischemic as well as ischemic cardiomyopathies will increase the population eligible to receive ICDs by as many as 500,000 patients. 62 (See Table 5.)
Electrophysiology Study
A referral for an electrophysiology consultation usually is indicated when patients have life-threatening or difficult to manage arrhythmias requiring interventional therapy, when benign but symptomatic arrhythmias cannot be controlled with medical therapy, when complicated invasive therapies such as radiofrequency ablation or ICD are being considered, or when initial antiarrhythmic measures have failed.
Indications have evolved dramatically over time, and the most recent guidelines provided by the ACC/AHA were published in 1995. EP studies are indicated for patients with unexplained syncope with evidence of structural heart disease on initial evaluation, for survivors of cardiac arrests that are not in the setting of an acute MI, for patients with unexplained palpitations prior to syncope, and for patients with palpitations accompanied by a rapid pulse as documented by a medical professional (Class I indications).59 Additional indications include VF arrest, sustained VT (or undiagnosed wide QRS complex tachycardia), symptomatic bradycardia, and device management or interrogation. Detailed discussions of these indications are outside of the scope of this paper.
Notably, the enthusiasm for electrophysiology studies as a tool to guide therapy in patients with prior myocardial infarction has waned considerably. The MADIT II trial, mentioned above, demonstrated that ICDs provided a significant mortality reduction (31%, p = 0.016) in patients without an EP study but with a prior myocardial infarction and ejection fraction less than 30%.59
Bradyarrhythmias
Bradyarrhythmias can be asymptomatic, but symptoms of lightheadedness, dizziness, presyncope or syncope, worsening of heart failure, or poor exercise tolerance may be seen. When a slow pulse is the cause for these symptoms and the problem is not reversible, a pacemaker is likely to be required.
A simple approach to differentiating bradycardias involves the relationship of P waves to the QRS complexes. If P waves are absent and the QRS complexes are irregular, slow AF or sinus arrest with irregular escape rhythm is most likely. If P waves are absent but the QRS complexes are regular, the diagnoses can include sinus arrest with regular escape rhythm or possibly AF with AV block. If a 1:1 relation exists between the P waves and the QRS complexes, possible etiologies include sick sinus syndrome or sinus bradycardia. If atrial flutter has been excluded and there are more P waves than QRS complexes, the diagnosis is second-degree or third-degree AV block.
Sinus Bradycardia
In normal hearts, sinus bradycardia can be asymptomatic and have rates as low as 30 bpm and pauses of up to 2 seconds during sleep without prognostic significance.63
Sinus bradycardia can be seen in a variety of pathological states as well, however. These may include infiltrative diseases, such as sarcoidosis or amyloidosis; infectious diseases, like Lyme carditis, endocarditis, and Chagas disease; or collagen vascular diseases. Potentially reversible causes of sinus bradycardia include medications, electrolyte abnormalities, hypothyroidism, and exaggerated vagal stimulation. Similar processes also can affect the atrioventricular (AV) node, bundle of His, or infra-His conduction system, causing AV block.
Sinus bradycardia may not require treatment in the absence of symptoms. For symptomatic patients, treatment should focus on evaluation for the source of the bradycardia. Medication lists should be reviewed carefully searching for medications that depress sinus node function. In symptomatic patients, beta-blockers should be carefully discontinued, clinical scenario permitting. Care must be exercised so as to not precipitate angina. Other reversible causes, such as hypothyroidism, should be excluded. Should these measures fail, temporary or permanent pacing should be considered to treat these patients. Acutely, intravenous atropine or isoproterenol may be employed to temporarily increase heart rate until pacing can be instituted.
Sick Sinus Syndrome
Sick sinus syndrome is a general term used to describe disordered electrical activity in the right atrium. Pathological studies in patients with sick sinus syndrome describe fibrosis of the sinus node, AV node, and right atrium64-66 leading to either disordered sinus impulse generation or propagation.67-69 Originally coined by Lown in 1967 to describe "chaotic atrial activity" in some patients following failed cardioversion for atrial fibrillation,70 the term has been expanded to include a spectrum of manifestations, including sinoatrial exit block, tachycardia-bradycardia syndrome, sinus arrest, chronic AF with slow ventricular response, and sinus bradycardia punctuated by recurrent paroxysmal AF or frequent atrial premature systoles.65,66,71-74 These arrhythmias should be correlated with symptoms to be called sick sinus syndrome.75
Sinus Arrest
Sinus arrest is defined by normal conduction of P waves that originate in the sinus, punctuated by sinus pauses of greater than 3 seconds. Clinical manifestations may vary widely, depending on the duration, from asymptomatic to fatigue to lightheadedness and syncope. The etiology can be difficult to determine. It may present in the setting of an acute infarct as a result of hypoperfusion. Other conditions, such as infiltrative diseases, may contribute to the development of fibrosis of the sinus node, which may contribute to the sinus arrest. Medications such as digoxin and beta-blockers may exacerbate sinus node disease. Electrolyte imbalances also may worsen sinus node function, and if found, may require investigation for endocrine causes.
In some patients, bradycardia and arrest may be related to vagal stimulation slowing the sinus rate and lengthening the refractory period of the sinus node. This finding has been observed in athletes. In carotid sinus syndrome (CSS), stimulation of one or both of the hypersensitive carotid sinuses at the bifurcation of the common carotid arteries produces brief episodes of faintness or loss of consciousness. One mechanism by which this takes place is a cardioinhibitory action in which vagally mediated bradycardia causes sinus arrest or atrioventricular block for more than 3 seconds.
Sinoatrial Exit Block
Sinoatrial exit block arises when pacemaker function is intact, but perinodal atrial tissue conducts abnormally. Like atrioventricular (AV) node block discussed below, sinoatrial exit block is categorized as first-, second-, or third-degree block. Only second-degree blocks have surface ECG findings. Progressive shortening of the PP interval followed by a non-conducted P wave defines second-degree, type I, sinoatrial block. A sinus pause without P waves that is a multiple of the PP interval is evidence for second-degree, Type II, sinoatrial block.
Tachycardia-Bradycardia Syndrome
Tachycardia-bradycardia syndrome (TBS) is present when an atrial tachyarrhythmia alternates with a bradyarrhythmia. Symptoms are caused by a reduction in cardiac output from either arrhythmia74,76,77 and can present as worsening heart failure or syncope, or more insidiously as memory or concentration difficulties.66 Paroxysmal AF is the most common tachyarrhythmia observed in patients with sick sinus syndrome;78 however, a number of tachy- and brady-arrhythmias can be seen. Patients with tachycardia-bradycardia syndrome have an increased risk for thromboembolism over other patients with sick sinus syndrome,79 particularly in association with AF.80
Often, the bradyarrhythmia in TBS reflects disease of the sinus node, expressed as sinoatrial exit block or sinus arrest. However, in some instances, the tachyarrhythmia itself may cause electrical remodeling of the sinoatrial node. Catheter ablation of AF in these cases may induce reverse remodeling of the SA node and improve bradycardia.81 This process is not likely a factor in the majority of cases, however, where pacemakers in combination with AV nodal blocking agents remain the mainstay of treatment.
Sick sinus syndrome is the most common indication for pacemaker implantation in the United States, accounting for approximately 50% of implantations from 1981 to 1997.82 Worldwide, sick sinus syndrome and AV block are approximately equally cited as the indications for pacemaker implantation.83 There is debate about the optimal pacemaker selection in patients with sick sinus syndrome. In patients with normal AV conduction, atrial pacing is preferred to ventricular pacing, as it improves survival from cardiac death, thromboembolic complications,84,85 and incidence of AF.77 These data may not be applicable to patients with more widespread conduction system disease, however. Three prospective randomized trials of physiologic dual chamber pacing vs. single chamber ventricular pacing in more general populations of patients with sinus node dysfunction have failed to show improved mortality or reduced incidence of stroke despite decreased incidence of AF in patients with dual chamber pacing.86-89
AV Blocks
Many of the pathologic processes that involve the sinus node also may affect the atrioventricular (AV) node. Several additional causes merit mention. Idiopathic fibrosis of the conduction system is a common cause of AV block.90-92 Lev's disease as originally described is "fibrosis of the left side of the cardiac skeleton," which includes the proximal bundles, aortic and mitral valves, and a portion of the septum.90 It generally affects older individuals, and can occur as an isolated finding. Lenegre's disease describes progressive fibrosis of the conduction system without involvement of other myocardium, usually in younger patients.91 In contrast, coronary artery disease is a relatively uncommon cause of permanent AV block.91,93-95 Drugs routinely used in patients with ischemic heart disease, such as beta-blockers, digitalis, and calcium channel blockers, may exacerbate AV block. AV block may manifest on physical exam as bradycardia or group beating. As with sinus bradycardia, symptoms can include exacerbation of heart failure, syncope, presyncope, dyspnea, or fatigue.
AV block is classified into first-, second-, and third-degree AV block based on ECG findings. Prolongation of the PR interval greater than 0.2 ms generally defines first-degree AV block. Surface ECG findings provide clues as to the origin of the block. Almost all first-degree AV blocks with a narrow QRS are due to block within the AV node, which should not require further therapy. Occasionally, block will occur in the conduction system below the level of the AV node, sometimes signaled by the presence of a bundle branch block. During a prospective study of 554 patients with bi- and trifascicular block, the incidence of developing complete heart block was less than 4% over the average follow up of more than 3.5 years.96
There are four categories of second-degree AV block. Type I second-degree AV block, or Wenckebach AV block, involves progressive prolongation of the PR interval until a P wave fails to conduct. Type I second-degree AV block may be seen in normal individuals, athletes, and in the elderly. In the majority of cases, the conduction block is in the AV node itself, which may predict a benign course. However, block in the His bundle or infra-His structure may present similarly. Surface ECG findings alone cannot reliably differentiate block in the AV node from block in the His bundle or infra-His structures, and pacemakers may be considered more strongly in patients with infranodal block.
Type II second-degree AV block, or Mobitz AV block, is characterized by dropped QRS complexes without progressive prolongation of the PR interval. The PR intervals during Type II (Mobitz) block are consistent. In the case of 2:1 AV block (two P waves per QRS complex) it is not possible to differentiate Type I and Type II second-degree AV block. Advanced AV block is present when the ratio of P waves to QRS beats exceeds 2:1.
Third-degree heart block, or complete heart block, is present when there is absence of AV conduction. The sinus rate exceeds the escape rate, and there is no evidence of conduction to the ventricle. Third-degree heart block is a cause of atrioventricular dissociation, but the two terms are not synonymous.
In addition to the processes already mentioned for other forms of heart block, there are several congenital and familial forms of third-degree heart block. The vast majority of third-degree block in fetuses and neonates with structurally normal hearts is due to neonatal lupus, a condition strongly linked to the presence of anti SSA/Ro and anti SSB/La antibodies in maternal serum.97,98 With presentation later in childhood, complete heart block attributed to previously unrecognized neonatal lupus is rare,98 although progression from first- and second-degree heart block is possible.99 Other causes may be related to congenital heart malformations themselves or as sequelae of corrective surgeries, familial forms of progressive heart block, or related to neuromuscular disorders.100
Atrioventricular dissociation can occur without complete heart block if the rate of the escape mechanism is greater than the sinus rhythm, as occasionally occurs in sinus bradycardia or accelerated idioventricular rhythm.
Pacemakers are the treatment of choice for patients with symptomatic AV block due to an irreversible cause, including AV block from ablation procedures or surgery, or as a result of neuromuscular disorders such as myotonic muscular dystrophy. Other Class I indications for permanent pacing include documented asystole of 3 seconds or more or an escape rate of less than 40 bpm in an awake patient, regardless of whether symptoms are present. In the setting of reversible causes of AV block, such as acute ischemia or electrolyte abnormalities, pacemakers usually are not required. EP studies may be useful in situations where bradycardia is suspected as the cause of symptoms, but no arrhythmia has been documented.101
The primary indication for pacemakers remains symptomatic bradycardia. In the absence of symptoms, many patients can be observed safely. Pacemakers may be indicated to manage bradycardia caused by medications when these medications cannot be discontinued. Further information regarding indications for pacemaker implantation is available at the ACC/AHA/HRS web site. The selection of specific pacemaker models and programming features is outside the realm of this paper.101
Conclusion
The clinical presentation and management of common arrhythmias has been reviewed. Prognosis of various arrhythmias has also been discussed, along with treatment options. Indications for inpatient management have been reviewed, as have indications for referral to a cardiologist or electrophysiologist. The most important part of the initial evaluation remains the history, physical examination, and the ECG. Often, the fear of missing a serious arrhythmia drives clinicians to order expensive and inappropriate tests. Correct identification of the arrhythmia early allows patient care to be delivered in a safe and cost-effective method.
References
1. Braunwald E, Zipes DP, et al. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia, WB Saunders, 2001.
2. Manolio TA, Furberg CD. Epidemiology of sudden cardiac death. In: Akhtar M, Myerburg RJ, Ruskin JN, eds. Sudden Cardiac Death: Prevalence, Mechanisms, and Approaches to Diagnosis and Management. Malvern, PA, Williams & Wilkins, 1994.
3. Barsky AJ, Cleary PD, Coeytaux RR, et al. Panic disorder, palpitations, and the awareness of cardiac activity. J Nerv Ment Dis 1994; 182:63-71.
4. Zimetbaum P, Josephson ME. Evaluation of patients with palpitations. N Engl J Med 1998;338:1369-1373.
5. Rosano GM, Leonardo F, et al. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet 1996;347(9004):786-788.
6. Shih-Huang Lee MD, Pan-Chen Chang MD, et al. Circadian variation of paroxysmal supraventricular tachycardia. Chest 1999;115:674-678.
7. Watanabe M, Nakagawa M, et al. Circadian variation of short-lasting asymptomatic paroxysmal supraventricular tachycardia. J Electrocardiol 2002;35:135-138.
8. Krahn AD, Klein GJ, Skanes AC, et al. Insertable loop recorder use for detection of intermittent arrhythmias. Pacing Clin Electrophysiol 2004;27:657-664.
9. Shen WK. Modification and ablation for inappropriate sinus tachycardia: Current status. Card Electrophysiol Rev 2002;349-355.
10. Lee RJ, Kalman JM, et al. Radiofrequency catheter modification of the sinus node for "inappropriate" sinus tachycardia. Circulation 1995;92:2919-2928.
11. Furlan R, Snell M, et al. Chronic orthostatic intolerance: A disorder with discordant cardiac and vascular sympathetic control. Circulation 1998;98:2154-2159.
12. Stewart JM. Pooling in chronic orthostatic intolerance: Arterial vasoconstrictive but not venous compliance defects. Circulation 2002;105:2274-2281.
13. Stewart JM. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation 2002;106:2946-2954.
14. Shen WK, Low PA, et al. Is sinus node modification appropriate for inappropriate sinus tachycardia with features of postural orthostatic tachycardia syndrome? PACE 2001;24:217-230.
15. Furberg CD, Psaty BM, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994;74:236-241.
16. Krahn AD, Manfreda J, et al. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995;98:476-484.
17. Diker E, Aydogdu S, et al. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am J Cardiol 1996;77:96-98.
18. Carson PE, Johnson GR, et al. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation 1993;87:VI102-110.
19. Woeber KA. Thyrotoxicosis and the heart. N Engl J Med 1992;327:94-98.
20. Mehta A, Jain AC, et al. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol 1997:273-283.
21. Ettinger PO, Wu CF, De La Cruz C Jr, et al. Arrhythmias and the "holiday heart." Alcohol associated cardiac rhythm disorders. Am Heart J 1978; 95:555.
22. Weber DM, Phillips JH Jr. A re-evaluation of electrocardiographic changes accompanying acute pulmonary embolism. Am J Med Sci 1966;251:381.
23. Maisel WH, Rawn JD, et al. Atrial fibrillation after cardiac surgery. Ann Intern Med 2001;135:1061-1073.
24. Kopecky SL, Gersh BJ. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med 1987;317:669-674.
25. Hart RG, Pearce LA, et al. Stroke with intermittent atrial fibrillation: Incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183-187.
26. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-2870.
27. Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA 2003;290:2685-2692.
28. Petersen P, Boysen G, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1989;1:175-179.
29. Connolly SJ, Laupacis A, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol 1991;18:349-355.
30. Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 1991;84:527-539.
31. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. N Engl J Med 1990;323:1505-1511.
32. Ezekowitz MD, Bridgers SL, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406-1412.
33. Wyse DG, Waldo AL, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825-1833
34. Van Gelder IC, Hagens VE, et al. Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002 ;347:1834-1840.
35. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): A randomised trial. Lancet 2000;356:1789-1794.
36. Singh BN, Singh SN ,et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005;352:1861-1872.
37. Swartz JF, Pellersels G, Silvers J, et al. A catheter-based curative approach to atrial fibrillation in humans. Circulation 1994;90:Suppl 1:I-335.
38. Wellens HJ. Contemporary management of atrial flutter. Circulation 2002;106:649-652.
39. Miller JM, Zipes DP. Catheter ablation of arrhythmias. Circulation 2002:106:e203-e205.
40. Morady F. Radiofrequency ablation as treatment for cardiac arrhythmias. N Engl J Med 1999;340:534-544.
41. Goodacre S, Irons R. Atrial arrhythmias. BMJ 2002:324(7337): 594-597.
42. Josephson ME. Clinical Cardiac Electrophysiology. 3rd ed. Philadelphia, Lippincott Williams & Wilkins, 2002.
43. Zipes DP, DiMarco JP, Gillette PC, et al. Guidelines for clinical intracardiac electrophysiological and catheter ablation procedures: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Intracardiac Electrophysiologic and Catheter Ablation Procedures), developed in collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol 1995;26:555-573.
44. Zimetbaum P. Inpatient or outpatient initiation of antiarrhythmic medications for atrial fibrillation. Available at www.americanheart.org/downloadable/heart/1023122768115zimetbaum_inpatient_vs_outpatient.pdf.
45. Slater W, Lampert S, Podrid PJ, et al. Clinical predictors of arrhythmia worsening by antiarrhythmic drugs. Am J Cardiol 1987;59:38E.
46. The Cardiac Arrhythmia Suppression Trial Investigators (CAST). Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial arrhythmia suppression after myocardial infarction. N Engl J Med 1989;10:406.
47. Simpson RJ Jr, Cascio WE, Schreiner PJ, et al. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002;143:535-540.
48. Julian DG, Camm AJ, Frangin G, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet 1997;349:667-674. Erratum in: Lancet 1997;349:1180. Lancet 1997;349:1776.
49. Cairns JA, Connolly SJ, Roberts R, et al. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997;349:675-682. Erratum in: Lancet 1997 Jun 14;349(9067):1776.
50. Janse MJ, Malik M, Camm AJ, et al. Identification of post acute myocardial infarction patients with potential benefit from prophylactic treatment with amiodarone. A substudy of EMIAT (the European Myocardial Infarct Amiodarone Trial). Eur Heart J 1998;19:85-95.
51. Akhtar M, Shenasa M, et al. Wide QRS complex tachycardia. Reappraisal of a common clinical problem. Ann Intern Med 1988;109:905-912.
52. Steinman RT, Herrera C, et al. Wide QRS tachycardia in the conscious adult. Ventricular tachycardia is the most frequent cause. JAMA 1989;261:1013-1016.
53. Stewart RB, Bardy GH, Greene HL. Wide complex tachycardia: Misdiagnosis and outcome after emergent therapy. Ann Intern Med 1986;104:766-771.
54. Brugada P, Brugada J, et al. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991;83:1649-1659.
55. Drew BJ, Scheinman MM. ECG criteria to distinguish between aberrantly conducted supraventricular tachycardia and ventricular tachycardia: Practical aspects for the immediate care setting. Pacing Clin Electrophysiol 1995;18(12 Pt 1):2194-2208.
56. Lau EW, Ng GA. Comparison of the performance of three diagnostic algorithms for regular broad complex tachycardia in practical application. Pacing Clin Electrophysiol 2002;25:822-827.
57. Baerman JM, Morady F, DiCarlo LA Jr, et al. Differentiation of ventricular tachycardia from supraventricular tachycardia with aberration: Value of the clinical history. Ann Emerg Med 1987;16:40-43.
58. Gupta AK, Thakur RK. Wide QRS complex tachycardias. Med Clin North Am 2001;85:245-266, ix-x.
59 Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-883.
60. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-2158.
61. Bardy GH, Lee KL, Mark DB, et al.. SCD-HeFT. The Sudden Cardiac Death in Heart Failure Trial. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-237.
62. Centers for Medicare and Medicaid Services Press Release. Medicare Collaborates To Help Doctors And Patients Use Implantable Defibrillators Effectively. October 27, 2005. Published online at http://www.cms.hhs.gov/media/?media=pressr.
63. Tresch DD, Fleg JL. Unexplained sinus bradycardia: Clinical significance and long term prognosis in apparently healthy persons older than 40 years. Am J Cardiol 1986;58:1009-1013.
64. Evans R, Shaw DB. Pathological studies in sinoatrial disorder (sick sinus syndrome). Br Heart J 1977;39:778-786.
65. Ferrer MI. The sick sinus syndrome in atrial disease. JAMA 1968;206:645-646.
66. Kaplan BM, Langendorf R, Lev M, et al. Tachycardia-bradycardia syndrome (so-called "sick sinus syndrome"). Pathology, mechanisms and treatment. Am J Cardiol 1973;31:497-508.
67. Wu DL, Yeh SJ, Lin FC, et al. Sinus automaticity and sinoatrial conduction in severe symptomatic sick sinus syndrome. J Am Coll Cardiol 1992;19:355-364.
68. Demoulin JC, Kulbertus HE. Histopathological correlates of sinoatrial disease. Br Heart J 1978;40:1384-1389.
69. Thery C, Gosselin B, Lekieffre J, et al. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. Am Heart J 1977;93:735-740.
70. Lown B. Electrical reversion of cardiac arrhythmias. Br Heart J 1967;29:469-489.
71. Rubenstein JJ, Schulman CL, et al. Clinical spectrum of the sick sinus syndrome. Circulation 1972;46:5-13.
72. Birchfield RI, Menefee EE, Bryant GD. Disease of the sinoatrial node associated with bradycardia, asystole, syncope, and paroxysmal atrial fibrillation. Circulation 1957;16:20-26.
73. Bouvrain Y, Slama R, Temkine J. [Sino-auricular block and "sinus diseases": Reflections apropos of 63 cases]. Arch Mal Coeur Vaiss 1967;60:753-773.
74. Bigger JT Jr, Reiffel JA. Sick sinus syndrome. Annu Rev Med 1979;30:91-118
75. Brignole M. Sick sinus syndrome. Clin Geriatr Med 2002;18:211-227.
76. Ferrer MI. The sick sinus syndrome. Circulation 1973;47:635-641.
77. Cosin J, Hernandiz A, et al. Sick sinus syndrome: Strategies for reducing mortality. Cor Vasa 1992;34:135-148.
78. Atlee, John L. MD. Perioperative cardiac dysrhythmias: Diagnosis and management. Anesthesiology 1997;86:1397-1424.
79. Kristensen L, Nielsen JC, et al. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual chamber pacing in 177 patients with sick sinus syndrome. Heart 2004;90:661-666.
80. Tse HF, Lau CP. Prevalence and clinical implications of atrial fibrillation episodes detected by pacemaker in patients with sick sinus syndrome. Heart 2005; 91:362.
81. Hocini M, Sanders P, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 2003;108:1172-1175.
82. Bernstein AD, Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol 2001;24:842-855.
83. Mond HG, Irwin M, Morillo C, et al. The world survey of cardiac pacing and cardioverter defibrillators: Calendar year 2001. Pacing Clin Electrophysiol 2004;27:955-964.
84. Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 1994;344:1523-1528.
85. Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick sinus syndrome. Lancet 1997;350:1210-1216.
86. Connolly SJ, Kerr CR, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 2000;342:1385-1391.
87. Lamas GA, Lee KL, et al. Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854-1862.
88. Stambler BS, Ellenbogen KA, et al. Pacemaker Selection in the Elderly Trial Investigators. Predictors and clinical impact of atrial fibrillation after pacemaker implantation in elderly patients treated with dual chamber versus ventricular pacing. Pacing Clin Electrophysiol 2003;26:2000-2007.
89. Kerr CR, Connolly SJ, et al. Canadian Trial of Physiological Pacing: Effects of physiological pacing during long-term follow-up. Circulation 2004;109:357-362.
90. Lev M. Anatomic basis for atrioventricular block. Am J Med 1964;37:742.
91. Lenegre J. Etiology and pathology of bilateral bundle branch block in relation to complete heart block. Prog Cardiovasc Dis 1964;55:409.
92. Rosenbaum MB, Elizari MV, Kretz A, et al. Anatomical basis of AV conduction disturbances. Geriatrics 1970;25:132-144.
93. Zoob M, Smith KS. The aetiology of complete heart-block. BMJ 1963;5366:1149.
94. Harris A, Davies M, Redwood D, et al. Aetiology of chronic heart block. A clinico-pathological correlation in 65 cases. Br Heart J 1969;31:206-218.
95. Davies MJ, Redwood D, Harris A. Heart block and coronary artery disease. Br Med J 1967;3:342-343.
96. McAnulty JH, Rahimtoola SH, Murphy E, et al. Natural history of ‘high risk' bundle-branch block: Final report of a prospective study. N Engl J Med 1982;307:137-143.
97. Buyon JP, Clancy RM. Neonatal lupus syndromes. Curr Opin Rheumatol 2003;15:535-541.
98. Jaeggi ET, Hamilton RM, Silverman ED, et al. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution's experience of 30 years. J Am Coll Cardiol 2002;39:130-137.
99. Askanase AD, Friedman DM, Dische MR, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus 2002;11:145-151.
100. Benson DW. Genetics of atrioventricular conduction disease in humans. Anat Rec A Discov Mol Cell Evol Biol 2004;280:934-939.
101. Gregoratos G, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation 2002;106:2145-2161.