Pediatric Sepsis and Septic Shock
February 1, 2018
Reprints
AUTHORS
Rasha D. Sawaya, MD, Assistant Professor of Clinical Emergency Medicine; Associate Program Director, Emergency Medicine Residency; Director of Pediatric Quality, Department of Emergency Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Imane Chedid, MD, Emergency Medicine Resident, American University of Beirut Medical Center, Lebanon
Imad El Majzoub, MD, Fellow, Emergency Medicine, MD Anderson Cancer Center, Houston, TX
PEER REVIEWER
Aaron Leetch, MD, Assistant Professor of Emergency Medicine & Pediatrics; Residency Director, Combined Emergency Medicine & Pediatrics Residency, University of Arizona College of Medicine, Tucson
EXECUTIVE SUMMARY
- Pediatric severe sepsis usually is community-acquired (57%) and occurs most often in toddlers (median age of 3 years with interquartile range, 0.7-11.0). The most common primary site of infection is the respiratory tract. Interestingly, one study noted the most common pathogen retrieved from blood cultures was Staphylococcus aureus.
- Severe sepsis occurs when there is sepsis and organ hypoperfusion or dysfunction, such as an elevated lactate, oliguria, prolonged capillary refill time, reduced mental status, disseminated intravascular coagulopathy, acute respiratory distress syndrome, or acute renal failure.
- The physical exam of a septic child may be as subtle as isolated tachycardia or as flagrant as hypotension or poor perfusion with an altered mental status.
- Systemic inflammatory response syndrome has a high specificity, but a poor sensitivity, for sepsis. One series showed an overall sensitivity of 31.2% (95% CI, 27.3-35.4%) and specificity of 95.7% (95% CI, 94.2-97%).
- Even a one-hour delay in the initiation of appropriate resuscitation measures in pediatric patients with sepsis
- was associated with increased mortality (OR, 2.29; 95% CI, 1.19-4.44).
- Start with a volume of 20 mL/kg within the first five minutes. This can be rapidly pushed in with 60 mL syringes or rapid infusers if available, or a three-way stop cock and push-pull system; using IV pumps may be too slow.
- The 2017 guidelines recommend starting with epinephrine for cold shock and norepinephrine for warm shock. Dopamine is a second-line agent.
- Early administration of antibiotics is crucial to decrease mortality rates in patients with severe sepsis or septic shock.
Pediatric sepsis is a high-stakes diagnosis that requires vigilance to make an early, timely diagnosis. Aggressive resuscitation, including fluids, antibiotics, and vasoactive agents, may be necessary. Rapidly changing standard of care also makes sepsis a critical diagnosis for clinicians.
— Ann Dietrich, MD, FAAP, FACEP, Editor
Epidemiology
Pediatric sepsis syndrome is a leading cause of morbidity and mortality, and results in elevated healthcare costs for infants and children worldwide.1,2 Morbidity and mortality from sepsis are related to the causes of systemic inflammatory response syndrome (SIRS), complications of organ failure, and the potential for prolonged hospitalization.1,2,3
According to data from the 2015 SPROUT study, the point prevalence of severe sepsis globally was 8.2% (95% confidence interval [CI], 7.6-8.9).4 In addition, mortality rates associated with sepsis and septic shock in patients admitted to the pediatric intensive care unit (PICU) were 5.6% and 17.0%, respectively.5
Pediatric severe sepsis usually is community-acquired (57%)6 and occurs most often in toddlers (median age of 3 years with interquartile range, 0.7-11.0).4 The most common primary site of infection is the respiratory tract.4 Interestingly, one study noted the most common pathogen retrieved from blood cultures was Staphylococcus aureus.4
Definition of Sepsis and Organ Failure in the Pediatric Population
Systemic Inflammatory Response Syndrome
SIRS occurs when the body’s inflammatory state is revved up in response to an insult. The SIRS adult criteria have been modified to produce a pediatric-specific definition. In children, SIRS includes two or more of the following, one of which must be an abnormal temperature or leukocyte count:7
- A rectal temperature > 38.5°C or < 36°C;
- Heart rate more than two standard deviations (SD) above the normal, or bradycardia in children older than 1 year of age (< 10th percentile for age);
- Respiratory rate more than two SD above normal (or pCO2 < 32 mmHg);
- Leukocyte count > 12,000 cells/mm3, < 4,000 cells/mm3, or > 10% band forms.
Sepsis
As per the 2017 Sepsis-3 guidelines, sepsis in adults no longer is based on the SIRS criteria, but now is defined as an infection with at least one organ dysfunction.8 Currently, the definition of sepsis in the pediatric population remains based on the SIRS criteria, as evidence for change is still weak. However, this may change in future guidelines.9 For example, one study showed that a child with two or more SIRS criteria still lacked sensitivity and specificity for sepsis, and using SIRS alone would miss one in eight patients with sepsis.10 However, in children younger than 18 years of age, sepsis still is defined as a SIRS response caused by an infection that may be suspected or definite, and the cause may be viral, bacterial, or fungal.
Severe sepsis occurs when there is sepsis and organ hypoperfusion or dysfunction, such as an elevated lactate, oliguria, prolonged capillary refill time (CRT), reduced mental status, disseminated intravascular coagulopathy (DIC), acute respiratory distress syndrome, or acute renal failure.11
Although not included in the definition of sepsis, hyperglycemia, altered mental status, high lactate, and a prolonged CRT are all highly suggestive of sepsis and, therefore, should be considered when evaluating a child for sepsis.11
Shock
Septic shock is sepsis with fluid refractory hypotension and signs of hypoperfusion.11 Shock can be cold or warm. Definitions of shock are shown in Table 1.12
Table 1. Definitions of Shock12 |
||||
Type of Shock |
Characteristics |
|||
Central
|
Peripheral Pulse |
Skin |
Pulse
|
|
|
Cold Shock |
> 3 seconds |
Decreased |
Cool Mottled |
Narrow |
|
Warm Shock |
< 3 seconds |
Bounding |
Warm |
Wide |
|
Source: Author created. |
||||
Organ Dysfunction
Clinically, organ dysfunction is an important component of sepsis. Table 2 shows the criteria for organ dysfunction.13
Table 2. Criteria for Organ Dysfunction13 |
|
Organ System |
Criteria for Dysfunction |
|
Cardiovascular |
Hypotension* OR Need for vasoactive drug to maintain blood pressure in the normal range OR Two of the following:
|
|
Respiratory |
PaO2/FiO2 < 300 OR PaCO2 > 65 or 20 mmHg over baseline OR Need for > 50% FiO2 to maintain oxygen saturation ≥ 92% OR Need for nonelective mechanical ventilation |
|
Neurologic |
Glasgow Coma Scale score ≤ 11 OR Acute change in mental status |
|
Hematologic |
Platelet count < 80,000/microliter OR A decline of 50% from the highest value recorded over the previous three days OR Disseminated intravascular coagulopathy |
|
Renal |
Serum creatinine ≥ 2 times upper limit OR Two-fold increase in baseline creatinine |
|
Hepatic |
Total bilirubin ≥ 4 mg/dL** OR Serum glutamic pyruvic transaminase > 2 times upper limit |
|
*Hypotension is defined as: < 5th percentile for age or systolic blood pressure < 2 standard deviations below normal for age ** Often a normal variant in newborns Source: Author adapted. |
|
Etiology and Risk Factors
By definition, sepsis and septic shock include an infectious source, which can be bacterial, fungal, or viral. The most common site of infection is the respiratory tract, followed by the bloodstream, with respiratory infections having the highest mortality rates.14
Among the pathogens, bacterial causes, such as S. aureus and methicillin-resistant S. aureus (MRSA), frequently are isolated in the blood cultures and are a rising culprit in the post-vaccination era.15,16,17
In addition, the prevalence of Streptococcus pneumoniae and Neisseria meningitides is decreasing. Gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae, are the most frequently identified organisms in urinary tract infections.17 Viruses, such as influenza, parainfluenza, and adenovirus, also can cause sepsis.18,19
Risk factors for pediatric sepsis and septic shock are similar to those in adults.16 (See Table 3.) Being younger than 1 month of age also is an important risk factor to recall, especially because newborns initially may appear normal on exam.20
Table 3. Risk Factors for Pediatric Sepsis16 |
|
|
Source: Author adapted. |
Pathophysiology
The pathophysiology of sepsis and septic shock is not understood precisely but is thought to involve a complex interaction between the pathogen and the host’s immune system. The normal physiologic response to localized infection includes activation of the host defense mechanisms, which results in an influx of activated neutrophils and monocytes, a release of inflammatory mediators, local vasodilation, increased endothelial permeability, and activation of coagulation pathways. These response mechanisms occur during septic shock, leading to diffuse endothelial disruption, vascular permeability and DIC, vasodilation, and thrombosis of end-organ capillaries. This results in the clinical presentation of specific organ injury or multi-organ failure. 21
Clinical Features
Given the high mortality of septic shock and the rapid organ deterioration, it is considered a time-critical emergency. As detailed above, sepsis is the presence of SIRS criteria with a probable infection, and septic shock is sepsis with fluid refractory hypotension and signs of hypoperfusion. However, unlike adults, previously healthy children with intact cardiovascular homeostatic mechanisms can compensate extremely well during hypoperfusion states and do so for relatively long periods; their signs and symptoms will reflect this.12 For instance, a child with severe sepsis may be only tachycardic at presentation, maintaining his/her blood pressure within normal ranges for a relatively long period. But if the compensated shock remains unrecognized and untreated, the child will decompensate suddenly with a drop in blood pressure, making recovery more difficult.
Keep in mind, not every child with fever will have a serious infection that leads to sepsis. However, delaying recognition and the management of a septic child will worsen the prognosis significantly; hence, early recognition is crucial.
History
The typical presentation varies with the age of the patient. Even cursory knowledge of the developmental stages of children will help determine variations in activity by age. In neonates and infants, any change from the patient’s normal behavior, such as somnolence, irritability, or hypoactivity, with or without a fever, raises the possibility of sepsis.22 It is important to ask parents about the child’s baseline activity and what differs. Febrile children will be slightly hypoactive; therefore, it is important to pinpoint the state and activity of the child with and without the fever. Older infants and children typically present with a fever and a localized source of infection.23 (See Table 4.)
Table 4. Suspected Source of Infection in Sepsis With Respective Signs and Symptoms23 |
|
Suspected Source |
Signs and Symptoms |
|
Upper respiratory tract |
Rhinorrhea, hoarseness, muffled voice, sore throat, dysphagia Pharyngeal inflammation plus exudate ± swelling and lymphadenopathy |
|
Lower respiratory tract |
Productive cough, pleuritic chest pain |
|
Urinary tract |
Fever, urgency, dysuria, loin or back pain, incontinence |
|
Genital tract |
Vaginal or urethral discharge, lower abdominal pain, scrotal pain |
|
Wound or burn |
Inflammation, edema, erythema, purulent discharge |
|
Skin/soft tissue |
Erythema, edema, lymphangitis |
|
Central nervous system |
Signs of meningeal irritation: neck stiffness, headache |
|
Gastrointestinal |
Abdominal pain, distension, diarrhea, and vomiting |
|
Joint |
Pain, warmth, decreased range of motion, limp, crepitus in necrotizing infections |
|
Source: Author adapted. |
|
Consider using the risk factors of sepsis from Table 3 as a guide to ask further questions about recent surgeries, recent hospitalization, and past medical history. For instance, the provider should inquire about recurrent infections, such as urinary tract infections, and chronic diseases, such as cystic fibrosis, splenic dysfunction, sickle cell disease, and congenital cyanotic heart diseases. It is important to look for the presence of immunodeficiency in children who have cancer or HIV, who are undergoing immunotherapy, who are taking chronic steroids, or who have severe malnutrition. Ask about the patient's vaccination status, with a focus on pneumococcal, Haemophilus, and meningococcal vaccination. In addition, the presence of a foreign body, such as an indwelling intravascular catheter, urinary catheter, or chest tube, increases the risk of infection.19-24
Physical Examination
Vital signs are crucial in identifying sick patients. In children, these vary by age.25 (See Table 5.) The physical examination findings of a septic child may be as subtle as isolated tachycardia or as flagrant as hypotension or poor perfusion with an altered mental status. Always consider sepsis, a differential of consequence, in children with persistently abnormal vital signs. Persistent tachycardia often is missed, as it may be attributed to fever or crying. Hypotension is a late finding in children; in this population, the diagnosis of shock cannot be based solely on the presence of the latter. However, hypotension in children with a suspected source of infection is confirmatory for the presence of septic shock.26 It is important to note that although Table 5 offers a normal range of vital signs in children, care needs to be taken when deciding that a child is hypotensive. Having a systolic blood pressure lower than the range does not automatically make a child hypotensive. Table 5 also shows the systolic blood pressure below which a child needs evaluation for hypotension. Also note the use of the term “persistent” for the tachycardia. This reflects the fact that tachycardia secondary to fever, pain, or crying will get better when the cause is treated and will not “persist.”
Table 5. Age-adjusted Range of Normal Vital Signs25 |
|||||
Age |
HR |
SBP |
Definition of Hypotension as per SBP |
DBP |
RR |
|
< 1 month |
110-160 |
65-85 |
< 60 |
45-55 |
35-55 |
|
1-3 months |
110-160 |
65-85 |
< 70 |
45-55 |
35-55 |
|
3-6 months |
110-160 |
70-90 |
50-65 |
30-45 |
|
|
6-12 months |
90-160 |
80-100 |
55-65 |
22-38 |
|
|
1-3 years |
80-150 |
90-105 |
< 70 + (age in years x 2) |
55-70 |
22-30 |
|
3-6 years |
70-120 |
95-110 |
60-75 |
20-24 |
|
|
6-12 years |
60-110 |
100-120 |
60-75 |
16-22 |
|
|
> 12 years |
60-110 |
110-135 |
< 90 |
65-85 |
12-20 |
|
HR: heart rate in beats per minute; SBP: systolic blood pressure in mmHg; DBP: diastolic blood pressure in mmHg; RR: respiratory rate in breaths per minute Source: Author adapted. |
|||||
Other physical exam signs suggestive of sepsis are included in Table 6.22 Physical exam findings also can help differentiate the type of septic shock. (See Table 1.) In cold shock, the child will have mottled skin and prolonged central CRT (> 3 seconds). Patients at this stage will be tachycardic yet still will maintain their blood pressure in the normal age-adjusted range. This type of shock is seen most often in infants and young children. It is due to myocardial hypocontractility along with compensatory peripheral vasoconstriction. 27 Warm shock is more common in older children, and the provider will note a shorter (flash) CRT, warm skin, and bounding pulses. This is due to peripheral vasodilation along with a compensatory high cardiac output state.
Table 6. Physical Exam Signs by Organ System22 |
|
Organ |
Sign |
|
Cardiovascular |
|
|
Respiratory |
Tachypnea, apnea (especially in infants), grunting, nasal flaring, hypoxia |
|
Mental status |
Sleepiness, lethargy, agitation, fussiness, acting abnormal per parents |
|
Source: Author created. |
|
The key take-home message is that a child with a suspected infection, persistently abnormal vital signs, or a concerning exam after antipyretics and intravenous (IV) fluids to treat dehydration should be investigated and treated for sepsis or admitted for close observation.
Diagnostic Evaluation
Indications for Specific Laboratory Evaluation
Whenever sepsis or septic shock is diagnosed based on the presentation (history and physical exam), laboratory studies can help determine the type and source of the infection as well as the potential organ damage endured and patient prognosis. Recommended tests are listed below.
Complete Blood Count With Differential. This test can reveal leukocytosis or leukopenia, thrombocytosis (since platelets are an acute inflammatory marker), or thrombocytopenia. In the latter, consider DIC and complete the workup to confirm its presence with elevation of prothrombin time, partial thromboplastin time, international normalized ratio, D-dimer, and decreased fibrinogen.
Glucose. The presence of hypoglycemia or hyperglycemia has been associated with poor short-term outcomes in multiple studies.28,29,30 Therefore, providers should recognize and correct an abnormal blood glucose level promptly. Hypoglycemia is the most prevalent because of the high metabolic demand in sepsis and the decreased oral intake due to the illness, especially in neonates. Neonates should receive maintenance fluid with dextrose. 31 Correct hyperglycemia to a goal of ≤ 180 mg/dL.31
Electrolytes. Several electrolyte derangements secondary to the underlying illness can accompany sepsis and septic shock. Among them are hyponatremia or hypernatremia from severe dehydration because of gastrointestinal losses or decreased oral intake; hypophosphatemia, hypocalcemia, and hypomagnesemia also may be present. Providers should pay attention to serum calcium. In cases of hypocalcemia, replete calcium to prevent any further decreases in myocardial contractility. The American College of Critical Care still recommends this practice despite acknowledging the absence of solid evidence.27
Anion Gap. Calculate the anion gap (AG) using the following formula: AG = Na+ - (HCO3- + Cl-). In children, an anion gap > 14 to 16 mEq/L is considered high, and in neonates a high anion gap is > 16 mEq/L.32,33 In septic children, the acid-base status varies. Patients might present with respiratory alkalosis due to tachypnea, or respiratory or metabolic acidosis. When metabolic acidosis is present, it is usually a high anion gap metabolic acidosis, due to lactic acidosis. If the anion gap is normal, look for other causes mimicking sepsis (e.g., renal tubular acidosis, certain drug ingestions, and hypernatremic dehydration).34
Urinalysis. The presence of pyuria, nitrites, or leukocyte esterase is suggestive of a urinary tract infection. 35
Blood Urea Nitrogen (BUN) and Creatinine. BUN would be elevated in the case of dehydration, and creatinine can reflect prerenal azotemia. However, a twofold increase in creatinine from baseline may indicate sepsis-induced kidney injury, a sign of end-organ hypoperfusion.36,37
Serum Total Bilirubin and Alanine Aminotransferase. A total bilirubin ≥ 4 mg/dL or alanine aminotransferase > 2 times the upper limit of normal for age indicates liver dysfunction in the setting of sepsis.13
Blood Gas (Arterial or Venous). A blood gas may assist with evaluation of three important factors: tissue oxygenation, adequacy of ventilation, and acid-base disturbances. At times, it can be unreliable to assess ventilation and oxygenation status by noninvasive methods, such as pulse oximetry, as it is affected by other factors such as weak pulses or cold extremities. Thus, an arterial blood gas (venous blood gas or capillary blood gas) in a nonhypotensive child will help detect impending respiratory failure and the need for invasive ventilation.38 An arterial blood gas, venous blood gas, or capillary blood gas also will help determine the type and severity of the acid-base derangement in a nonhypotensive child.39
Microbiology. When possible, draw the cultures before initiating antibiotic therapy but do not delay antibiotics in a critical child; all patients require a blood culture. The other cultures depend on the age of the child, the presentation, and the suspected source of infection. For example, all children younger than 3 months of age with septic shock need a full septic workup that includes blood, urine, and cerebrospinal fluid cultures. Do not delay antibiotics if the child is unstable for a lumbar puncture. Send a deep tracheal aspirate on patients with tracheostomies, and send a wound culture if cellulitis, abscess, or surgical wound is noted. Fungal cultures may be helpful in immunocompromised patients. Among other microbiology investigations, consider diagnostic serologic testing, such as viral culture, polymerase chain reaction, rapid immunoassay antigen test, or direct and immunofluorescent antibody staining to establish the source of infection when herpes simplex virus, enterovirus, or influenza infection is suspected. Viruses are a common cause of sepsis, with high rates of mortality for influenza.23-40 When available, consult an infectious disease team early to help with investigation and antimicrobial therapy.
Lactic Acid. When there is insufficient delivery of oxygen to the tissue, such as with hypoperfusion in sepsis and septic shock, aerobic metabolism will shift to anaerobic to continue the generation of adenosine triphosphate, for cellular survival. This anaerobic mechanism will lead to the generation of a byproduct: lactate. The normal range of lactate in children is 0.5 to 2.2 mmol/L,41 and an elevated lactate level can be an indicator of sepsis. In fact, multiple studies in children with sepsis or septic shock have shown the association between high lactate levels and mortality or poor outcome in sepsis. However, data regarding its use as a diagnostic tool still are sparse in the pediatric population; most studies show its value as a prognostic indicator. One prospective study in children with undifferentiated SIRS showed that a high lactate level of > 4 mmol/L was associated with a relative risk of 5.5 (95% CI, 1.9-16.0) of developing organ dysfunction within 24 hours.42 In another study evaluating the predictive value of blood lactate and in-hospital mortality, the odds for in-hospital mortality increased by 38% for every 1 mmol/L increase in blood lactate (odds ratio [OR], 1.38; 95% CI, 1.30-1.46; P < 0.001).43
In 2017, Sitaraman et al reported that the mean lactate levels were significantly higher in non-survivors than survivors (5.12 ± 3.51 vs. 3.13 ± 1.71 mmol/L; P = 0.0001). Specifically, a lactate level ≥ 4 mmol/L at admission to the PICU was a predictor of mortality (OR, 5.4; 95% CI, 2.45-12.09). If the lactate did not decrease by more than 10%, patients had a greater risk of mortality (likelihood ratio, 2.83; 95% CI, 1.82-4.41).44 Another study showed that serum lactate normalization, but not rate of clearance, was associated with a decrease in organ dysfunction (relative risk [RR], 0.46; 95% CI, 0.29-0.73; adjusted RR, 0.47; 95% CI, 0.29-0.78).45 It is noteworthy that most studies on lactate in children are done outside of the emergency department (ED) in the PICU.
Procalcitonin. Procalcitonin is a polypeptide prohormone of calcitonin. In the healthy population, the serum level is undetectable, but it is increased when there is a bacterial infection, probably as a result of bacterial endotoxins, making procalcitonin not only useful in detecting sepsis, but also in differentiating bacterial from viral infection.46,47, 48 Published data on its clinical use, especially in the emergency department, are promising. Procalcitonin appears to be a better indicator of serious bacterial infections compared to white blood cell count, absolute neutrophil count, and percent neutrophils,49,50 and of better use in children, as it is not age dependent.51 In addition, serum procalcitonin appears to be a better predictor of poorer outcome than C-reactive protein and neutrophil count in septic children. Elevated levels correlate with the presence of multiorgan dysfunction (P = 0.0001) and shock (P = 0.003).51
Indications for Specific Radiological Evaluation
The radiological evaluation is tailored to the clinical scenario. Providers should consider a chest radiograph for the child with respiratory symptoms, abnormal lung findings, or a white blood cell count > 20,000 cells/mm3.52 Abdominal imaging should be obtained for the child with a concern of an intra-abdominal process, such as appendicitis. Consider a brain computed tomography scan for children with an altered level of consciousness or new-onset seizures; in addition, DIC from sepsis may predispose to intracranial bleeds. Cardiac echocardiography should be considered in children with a new murmur or other signs of endocarditis, such as Osler nodes and splinter hemorrhages, or for those who develop signs of cardiogenic shock (cardiomegaly, hepatomegaly, and respiratory failure). If osteomyelitis or a septic joint is suspected based on physical exam findings of a limp, swollen, or stiff joint, consider radiographs, ultrasound, bone scan, or magnetic resonance imaging.
Differential Diagnosis
Although sepsis requires early recognition and treatment, in children some of the SIRS criteria, such as tachycardia and tachypnea, may have other causes. SIRS has a high specificity but a poor sensitivity for sepsis. One series showed an overall sensitivity of 31.2% (95% CI, 27.3-35.4%) and specificity of 95.7% (95% CI, 94.2-97%).53 Benign causes of tachycardia include fear, fever, and pain. Evaluate the child in the parent’s arms, allow the child to calm down, or keep the child on a monitor and leave the room to obtain more accurate vital signs. Tachycardia and tachypnea also typically are associated with fever. If the child is not showing any other signs of sepsis or septic shock, such as poor perfusion, then treat the fever with an antipyretic and reevaluate the child. Finally, tachycardia may be a sign of pain. Something as simple as acute otitis media may be extremely painful in a child. Treat the pain and reevaluate as above.
Tachycardia also may be secondary to dehydration. Carefully assess for indicators of dehydration, especially in children with gastrointestinal losses, such as decreased tears and urine output. Consider an intravenous fluid bolus to rehydrate the patient and reevaluate the tachycardia while closely observing for other signs and symptoms of sepsis and septic shock. Pneumonia also may present with tachypnea and tachycardia. Keep in mind that pneumonia also may be the source of infection in a septic patient.
Furthermore, myocarditis also should be on the differential of a persistently tachycardic child. Frequently reassess the response to fluid and monitor for crackles, hepatomegaly, or other signs of fluid overload. Most importantly, not every state of shock is due to sepsis. There are four types of shock leading to tissue hypoperfusion and end-organ damage: distributive, cardiogenic, hypovolemic, and obstructive.54 (See Table 7.) Sepsis is included in distributive shock. Sometimes, the initial clinical presentation makes it difficult to rapidly differentiate the types of shock in the ED. History, physical exam, and frequent reassessments are key when determining response to treatment.
Table 7. Four Categories of Shock and Their Respective Causes54 |
|
|
Shock |
Associated Etiologies |
|
Distributive |
Anaphylaxis, sepsis |
|
Cardiogenic |
Sepsis, brady- or tachyarrhythmia, myocarditis, cardiomyopathy, congenital heart disease |
|
Hypovolemic |
Gastrointestinal loss, hemorrhage, burns |
|
Obstructive |
Cardiac tamponade, tension pneumothorax, ductal dependent congenital cardiac lesions, massive pulmonary embolism |
|
Source: Author created. |
|
Finally, while following the septic shock guidelines, consider endocrine causes of persistent shock, such as adrenal insufficiency or hypothyroidism, and other findings, such as pneumothorax, intra-abdominal hypertension, or abdominal compartment syndrome, as a reason for persistent hypotension.27
Treatment of Septic Shock
Early Recognition
As in adults, early recognition of sepsis and septic shock is crucial to improving outcomes.55 Even a one-hour delay in the initiation of appropriate resuscitation measures in pediatric patients with sepsis was associated with increased mortality (OR, 2.29; 95% CI, 1.19-4.44).56 However, there are conflicting data, and more research in this area is warranted. For example, in a large longitudinal study, there was a clear benefit of implementing a quality intervention bundle focused on recognition of pediatric sepsis and timely antibiotic and fluid administration.57 In fact, mortality was five times higher in children who did not receive bundle-compliant care (OR, 5.0; 95% CI, 1.9-14.3) compared to those who did (OR, 0.20; 95% CI, 0.07-0.53).57
However, in a recent meta-analysis published in the New England Journal of Medicine, the researchers reported that children with sepsis who received early goal-directed therapy had no improvement in 90-day mortality (OR, 0.97; 95% CI, 0.82-1.14; P = 0.68) and it was associated with increased healthcare costs.58
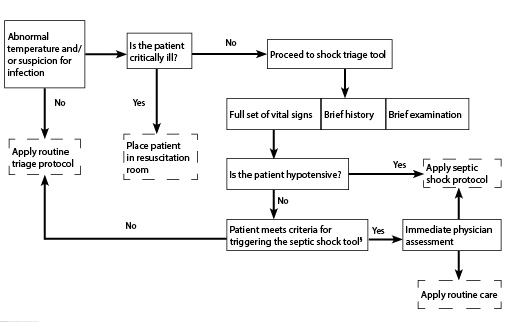
Currently the American College of Critical Care Medicine Guidelines emphasize early recognition and the implementation of a sepsis recognition bundle exemplified by the “septic shock identification trigger tool” shown in Figure 1. It is recommended that this bundle contain a trigger tool, rapid clinical assessment of the child, and initiation of the therapeutic approach. 27
Figure 1. Example of a Trigger Tool for Early Septic Shock Recognition27 |
||
 |
||
|
§ Apply the Septic Shock Protocol if:
|
||
|
High-risk Conditions
|
Septic Shock Checklist
|
|
|
Source: Author adapted. |
||
However, as acceptance and implementation of pathways is site specific, it is recommended to create a home-built bundle, adapted to the structure, staffing, equipment, and metrics of each institution.27
Early Goal-directed Therapy
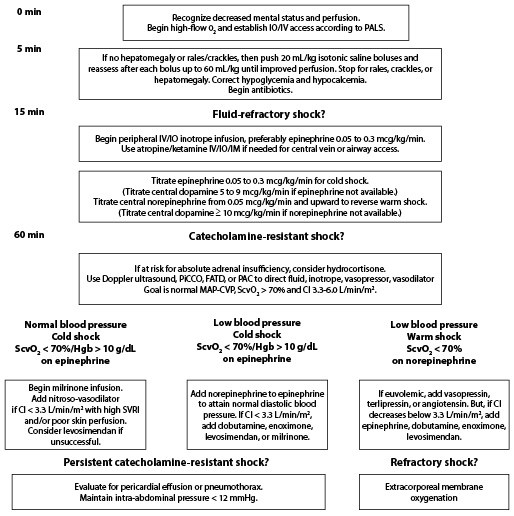
Once a child is identified as being in septic shock, follow the pediatric advanced life support (PALS) resuscitation algorithm shown in Figure 2.27 It is important to initiate IV/intraosseous (IO) access and fluid resuscitation within the first five minutes of recognition. Aim for early antibiotic administration, and tailor the inotropes or vasopressors as the clinical scenario mandates. These specific therapeutic interventions and the evidence behind them are detailed below.
Figure 2. American College of Critical Care Medicine Algorithm for Time-sensitive, Goal-directed Stepwise Management of Hemodynamic Support in Infants and Children27 |
 |
|
PiCCO: Pulse index Contour Continuous Cardiac Output (Pulsion Medical Systems, Germany); FATD: femoral artery thermodilution; PAC: pulmonary artery catheter; SVRI: systemic vascular resistance index. Adapted with permission from: Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med 2017;45:1061-1093. |
Time 0-5 Minutes: Vascular Access and Oxygen Therapy
Vascular Access. Initiate IV access within five minutes of recognition of sepsis or septic shock. If possible, place a minimum of two large-bore, free-flowing IV catheters. These depend on the age and size of the child, but aim for at least a 20 G needle if possible (the larger the better). At least two individuals should attempt these at the same time, one on each side. Consider looking at the child’s feet and scalp for veins.
If the IV is not in place after two attempts or 90 seconds in the setting of severe septic shock, insert an IO needle.59 Ultrasound-guided peripheral access also may be helpful in patients for whom IV access is difficult to establish. Do not delay care for central line placement; resuscitation can be done via peripheral or IO access adequately.
Oxygen Therapy. Provide supplemental oxygen immediately via a 100% non-rebreather face mask. If the patient is in respiratory distress, consider high-flow nasal cannula or noninvasive positive pressure ventilation. This will help increase oxygen content in the blood and delivery to the already poorly perfused tissues.11 Thereafter, closely monitor the oxygenation and work of breathing of the child. (See section below on mechanical ventilation.)
Time 5 to 15 Minutes: Fluid Resuscitation
A critical aspect of resuscitating a septic child is to replete the patient's intravascular volume. Evidence still is lacking regarding the choice of the proper solution, but crystalloids, such as normal saline and Ringer’s lactate, are equally effective as colloids, yet cheaper than the latter.31-60
While laboratory tests are being drawn and antibiotics prepared, the child requires fluid resuscitation. Infuse up to 60 mL/kg of isotonic fluids in the first 15 to 60 minutes of recognition of shock. Start with a volume of 20 mL/kg within the first five minutes. This can be rapidly pushed in with 60 mL syringes or rapid infusers if available; using IV pumps may be too slow. Using a three-way stop cock to create a push-pull system can allow rapid drawing and pushing of fluid.
It is crucial to monitor the response to fluid therapy after each bolus: Look for an increase in blood pressure, drop in heart rate, improved peripheral pulses and capillary refill, increased urine output, and level of consciousness. If there is no or little improvement, administer another bolus of 20 mL/kg of fluid, until reaching a total of 60 mL/kg over an hour.
It is noteworthy that a 2012 large, multicenter, multinational sub-Saharan study (the FEAST trial) showed a worse outcome with aggressive fluid resuscitation. This was thought to be due to the inability to deal with complications of fluid therapy as a result of a lack of infrastructure and technical support as well as to high rates of anemia and malnutrition.61 However, this study has raised questions about the paradigm of aggressive fluid resuscitation, calling for more studies. In the meantime, in low-resource settings or settings in which mechanical ventilation or pediatric intensive care may be delayed, use caution with fluid resuscitation.
In addition, neonates younger than 30 days of age and children with cardiac or renal disease with septic shock warrant less aggressive therapy, such as 5 to 10 mL/kg boluses. Because of the above data and the differential of septic shock previously discussed, which also includes cardiogenic shock, pay close attention to complications of fluid overload, such as crackles in the lungs or hepatomegaly, by reassessing for these after every bolus.
Time 15 to 60 Minutes: Fluid Refractory Shock and the Need for Vasoactive Medications
When a child remains in the state of shock after 60 mL/kg and rapid (over 15 to 60 minutes) fluid resuscitation, the patient is diagnosed with fluid refractory shock (i.e., septic shock). At this point, vasoactive drips should be started.
Understanding the mechanism of action of the different cardiovascular agents and on which receptors they work can help identify which one may be more suitable for the different types of septic shock and end-effect desired.27-62 (See Tables 8 and 9.) For example, norepinephrine has a more direct effect on peripheral vasculature than dopamine, dobutamine, and epinephrine, and, hence, is more potent in reversing hypotension in vasodilatory (warm) shock. Experts also recommend norepinephrine’s use when there is low systemic vascular resistance clinically seen as wide pulse
pressure with diastolic blood pressure < 50% of the systolic pressure.63,64
Table 8. Mechanism of Action of Different Cardiovascular Agents 27 |
|||
Cardiovascular Agent |
Receptor |
Action |
Use |
|
Dopamine |
β1 & 2 α: at higher doses (> 15 mcg/kg/min) |
Increased CO Vasoconstriction at higher dose |
Low CO state with adequate or increased SVR |
|
Dobutamine |
β1 & 2 |
Increased CO Arrhythmogenic |
Poor cardiac contractility with adequate or increased SVR |
|
Epinephrine |
β1 & 2 α: at higher doses (> 0.3 mcg/kg/min) |
Increased CO Vasoconstriction at higher dose |
Cold shock |
|
Norepinephrine |
α β1 & 2 at high doses |
Vasoconstriction Little effect on CO |
Warm shock, vasodilatation, and low SVR |
|
SVR: systemic vascular resistance; CO: cardiac output; β1-adrenergic receptor: found in cardiac smooth muscle and causes increased contractility; β2-adrenergic receptor: found in vascular smooth muscles (predominantly), cardiac smooth muscle (less), and lungs (bronchodilation) with its main effect being vasoconstriction. α-adrenergic receptor: found in arterial smooth muscle and causes vasoconstriction with increased venous return. Source: Author adapted. |
|||
Table 9. List of Inotropes/Vasopressors and Dosages62 |
||
Medication |
Dose |
Comments |
|
Dobutamine |
2.5 to 15 mcg/kg/min IV |
|
|
Dopamine |
Low dose: 2 to 5 mcg/kg/min IV Intermediate dose: 5 to 15 mcg/kg/min IV High dose: > 20 mcg/kg/min IV |
Low dose: minimal effect on heart rate and cardiac output Intermediate dose: starts ionotropic effect Titrate to effect |
|
Epinephrine |
0.1 to 1 mcg/kg/min |
Titrate to effect |
|
Milrinone |
50 mcg/kg IV bolus over 15 min, followed by a continuous infusion of 0.25 to 0.75 mcg/kg/min IV and titrate to effect |
Note: to avoid hypotension, some experts avoid giving a bolus while others divide the bolus into five aliquots and administer each aliquot over 10 min27 |
|
Norepinephrine |
Child: Start at 0.05 to 0.1 mcg/kg/min IV |
Max. dose: 2 mcg/kg/min IV Titrate to effect |
|
Source: Author adapted. |
||
Ionotropic agents, such as dopamine, dobutamine, and epinephrine, are the drugs of choice when there is depressed cardiac contractility. In addition, dopamine and epinephrine at high dosage (> 15 mcg/kg/min and > 0.3 mcg/kg/min, respectively) exert a sympathomimetic effect and a vasoconstrictive effect as well.
The 2017 guidelines recommend starting with epinephrine for cold shock and norepinephrine for warm shock.27 (See Figure 2.) Dopamine is a second-line agent.27 In fact, two recent publications described a decreased mortality and improved outcomes with the use of epinephrine as a first-line treatment in cold shock.63,64 Ramaswamy et al compared epinephrine to dopamine in pediatric septic shock and showed that epinephrine is better than dopamine for treatment of cold shock, with an odds ratio (OR) of shock resolution in the first hour equal to 4.8 (95% CI, 1.3-17.2; P = 0.019 ).65 In another study, dopamine drip was associated with higher mortality (OR, 6.5; 95% CI, 1.1-37.8; P = 0.037) and healthcare-associated infections (OR, 67.7; 95% CI, 5.0-910.8; P = 0.001) compared to epinephrine.66
Vasopressor support is a dynamic process. The first choice of vasopressor may need to be adjusted as the patient’s response is evaluated.
Finally, some authors suggest the use of vasodilatory agents, such as nitroprusside and type III phosphodiesterase inhibitors (PDEIs), like milrinone and inamrinone, when there is high systemic vascular resistance and low cardiac output, in addition to inotropes. PDEIs have a long half-life (1 to 10 hours) depending on the clearance and preferably are infused via central venous lines. These drips may lower the blood pressure; this typically responds to small boluses of fluids (5 mL/kg) and immediate discontinuation of the drug.27
Guidelines recommend administration of vasoactive agents through a central venous line when possible.27-31 However, if properly diluted, these agents, including epinephrine (e.g., 1 mg/50 mL), can be infused via a peripheral line to avoid delay in care. If extravasation of epinephrine occurs, treat with 1 to 5 mg of phentolamine diluted in 5 mL of normal saline.27
60 Minutes and Beyond: Catecholamine-resistant Shock
At this point, the patient ideally has been moved to a PICU. However, this often is not the case. At this point in time, central venous access should be started if not yet in place to aim for more specific and objective goal-directed therapy. Consider other causes of shock, such as pneumothorax, pericardial tamponade, or endocrine emergencies, if no improvement is noted and treat as identified.27-31
Add corticosteroids if a suspected or proven absolute adrenal insufficiency is noted, although mortality may not change.27-67 Use a hydrocortisone IV infusion at 50 mg/m2/24 hours.27 Consult the PICU and extracorporeal membrane oxygenation teams if available or start transfer to a hospital that has these resources.
Other Treatments to Provide Throughout the Golden Hour (60 minutes) of Care
IV Antibiotics. The Surviving Sepsis Guidelines stress the importance of antibiotic administration within one hour of sepsis recognition.11 Early administration of antibiotics is crucial to decrease mortality rates in patients with severe sepsis or septic shock. In one study, the mortality increased significantly with every one-hour delay in administration of antibiotics, but only after three hours delay from the initial dose. Specifically, for patients with more than a three-hour delay to initial and first appropriate antimicrobials, the OR for PICU mortality was 3.92 (95% CI, 1.27-12.06) and 3.59 (95% CI, 1.09-11.76), respectively.68 Therefore, do not delay the administration of antibiotics. Interestingly, however, recent adult data regarding time to antibiotics are mixed. A 2015 meta-analysis of adult patients did not show any benefit to early antibiotic treatment,69 yet a 2017 multicenter study of more than 40,000 adult patients showed that early antibiotic infusion rather than time to fluid resuscitation was associated with lower in-hospital mortality.70 Therefore, although it would be best to have two IV or IO lines for fluids and antibiotics, it may be acceptable to hold the completion of the fluid bolus in order to infuse the antibiotic, if a second access could not be placed. Remember, that many antibiotics also can be given intramuscularly if needed.
Start with a broad-spectrum carbapenem (e.g., meropenem, imipenem/cilastatin, or doripenem) or extended-range penicillin/β-lactamase inhibitor combination (e.g., piperacillin/tazobactam or ticarcillin/clavulanate).11 Several third- or higher-generation cephalosporins also can be used, especially as part of a multidrug regimen. Ceftriaxone plus vancomycin is widely available and easy to use and will provide wide Gram-negative and Gram-positive coverage, respectively. Always keep in mind all possible pathogens and the anticipated local microbial resistance. The following points may help guide the antibiotic choice:
- Give vancomycin to all patients with septic shock because of resistant organisms (e.g., MRSA).
- Consider the child’s age: Children younger than 1 month of age need Listeria monocytogenes, group B Streptococcus, and Gram-negative bacteria coverage, such as ampicillin and a third-generation cephalosporin (e.g., cefotaxime) or an aminoglycoside (e.g., gentamicin). A third-generation cephalosporin, such as ceftriaxone and vancomycin, may be enough for children older than 1 month of age to cover for N. meningitides and resistant S. pneumoniae and H. influenzae. Herpes simplex virus also may present solely as sepsis in neonates. Therefore, start acyclovir early while awaiting PCR results, especially if the infant had a seizure or has elevated liver enzymes.11
- Review previous positive cultures (e.g., in children with recurrent urinary tract infections), as they may show a resistance pattern to help guide antibiotic selection.
- The presence of a central line or immunosuppression predisposes the patient to Gram-negative bacteremia as well as fungemia, and will require more Gram-negative coverage, such as piperacillin/tazobactam. Consider ordering fungal cultures, especially in patients with recurrent or prolonged fevers. However, to date there is no evidence to recommend starting antifungal treatments in the ED.
- Site of the infection: If the source is a skin infection, consider adding MRSA coverage with clindamycin and vancomycin. If the source is in the feet, add Pseudomonas aeruginosa coverage with a beta-lactam and either an aminoglycoside or fluoroquinolones. Pneumonia with empyema is also suspicious for MRSA. If there is a gastrointestinal source, add anaerobic coverage such as piperacillin/tazobactam, clindamycin, or metronidazole.
- If toxic shock syndrome is suspected, add clindamycin for toxin neutralization.8
- Consider the season: During influenza season, add antiviral medications, such as oseltamivir.
- In travelers, review the provenance and consider treating for malaria and dengue fever if from an endemic zone. The Centers for Disease Control and Prevention website (www.cdc.gov) may help guide these decisions.
Finally, consult with the infectious disease team early to help with the antibiotic choice. See Table 10 for the dosage of all above listed antibiotics and antivirals.
Table 10. Antibiotic and Antiviral Dosages62 |
|
Medication |
Dose |
|
Acyclovir |
Immunocompetent: < 3 months of age and < 35 weeks gestational age: 40 mg/kg/24 hr IV divided Q12 h < 12 years of age: 60 mg/kg/24 hr IV divided Q8 h > 12 years of age: 30 mg/kg/24 hr IV divided Q8 h Immunocompromised: All ages: 750 to 1500 mg/m2/24 hr IV divided Q8 h |
|
Amphotericin, liposomal |
3 to 5 mg/kg/24 hr IV once daily |
|
Ampicillin |
< 7 days of age: 200 to 300 mg/kg/24 hr IV divided Q8 h > 7 days of age: 300 mg/kg/24 hr IV divided Q4-6 h |
|
Cefotaxime |
< 7 days of age: 100 to 150 mg/kg/24 hr IV divided Q8-12 h > 7 days of age: 150 to 200 mg/kg/24 hr IV divided Q6-8 h (> 12 years or ≥ 50 kg) and adults: 1 to 2 g/dose Q6-8 h IV Max. dose: 12 g/24 hr IV |
|
Ceftriaxone |
100 mg/kg/24 hr IV divided Q12 h Max. dose: 2 g/dose and 4 g/24 hr |
|
Clindamycin |
Neonate: 15 to 20 mg/kg/24 hr IV divided Q6-8 h Child: 20 to 40 mg/kg/24 hr IV divided Q6-8 h |
|
Gentamicin |
Neonate: 4 to 5 mg/kg/dose IV Child: 7.5 mg/kg/24 hr IV divided Q8 h Adult: 3 to 5 mg/kg/24 hr IV divided Q8 h |
|
Imipenem + Cilastatin |
< 1.2 kg or < 1 week of age: 50 mg/kg/24 hr IV divided Q12 h > 1.2 kg and > 1 week of age: 75 mg/kg/24 hr IV divided Q8 h >1 month of age: 60 to 100 mg/kg/24 hr IV divided Q6 h Max. dose: 4 g/24 hr |
|
Meropenem |
Neonate: Consult pharmacy > 3 months of age and child: 120 mg/kg/24 hr IV divided Q8 h |
|
Metronidazole |
< 7 days of age: < 1.2 kg: 7.5 mg/kg/dose IV Q48 h 1.2 to 2 kg: 7.5 mg/kg/dose IV Q24 h ≥ 2 kg: 15 mg/kg/24 hr IV divided Q12 h ≥ 7 days of age: < 1.2 kg: 7.5 mg/kg IV Q24 h 1.2 to 2 kg: 15 mg/kg/24 hr IV divided Q12 h ≥ 2 kg: 30 mg/kg/24 hr IV divided Q12 h > 1 month, child, or adult: 30 mg/kg/24 hr IV divided Q6 h Max. dose: 4 g/24 hr |
|
Oseltamivir |
< 3 months of age: 12 mg PO BID 3 to 5 months of age: 20 mg PO BID 6 to 12 months of age: 25 mg PO BID If > 1 year of age, weight adjusted: < 15 kg: 30 mg PO BID 15 to 23 kg: 45 mg PO BID 23 to 40 kg: 60 mg PO BID > 40 kg: 75 mg PO BID |
|
Piperacillin/Tazobactam |
100 mg piperacillin/kg/dose IV Q8 h Max. dose: 16 g piperacillin/24 hr |
|
Vancomycin |
Neonates: Consult pharmacy Infant/child: 60 mg/kg/24 hr IV divided Q6 h Max. dose: 1 g/dose |
|
Source: Author adapted. |
|
Mechanical Ventilation. The decision to intubate is based on clinical judgment. Beware of apnea, increased work of breathing, or decreased level of consciousness with inability to protect the airway. A Glasgow Coma Scale score < 8 or one that is rapidly deteriorating is an indication to intubate. Consider the following factors: When a child is anticipated to receive very large volumes of fluid during resuscitation > 60 mL/kg,71 remember that young infants have smaller functional residual capacity in the lungs and may require earlier intubation.72 Also, intubation in severe septic shock decreases the body’s demand on lung perfusion and helps divert perfusion to other organs.
Watch the child closely if intubating. The start of positive pressure ventilation in addition to sedatives will decrease the venous return and preload further and risk precipitating cardiovascular collapse and cardiac arrest. If possible, only intubate when the child already has received adequate fluid resuscitation and is on or starting inotropic support. Otherwise, have these at the bedside for immediate administration if needed.27
Avoid the use of etomidate for sedation. Several studies, including a 2012 meta-analysis, have shown etomidate to be harmful in pediatric patients with septic shock.73 A slow push of ketamine (0.25 to 1 mg/kg)62 unless otherwise contraindicated is a good alternative sedative, especially since it has relatively stable hemodynamics.
Other Therapeutic Options. Another important consideration for fluid therapy is the use of blood products. Guidelines recommend an initial target of 10 g/dL of hemoglobin as in adults, which changes to > 7 g/dL once the patient is stabilized.27
Therapeutic Endpoints
The American College of Critical Care Medicine and PALS emphasize maintaining or restoring good perfusion, adequate heart rate for age, and good respiratory support as in airway, oxygenation, and circulation within the first hour of shock recognition.27
Sepsis and septic shock resuscitation aim to reach specific therapeutic endpoints discussed in the previous sections and listed below.
Noninvasive Methods. Very simple measures can guide the provider’s approach to the septic child. Data have shown that a simple combined assessment of heart rate, CRT, and systolic blood pressure is a reliable indicator of shock in children. 74 Reassess the patient frequently after each treatment. Monitor the heart rate. A decrease in the heart rate suggests an improvement in the intravascular volume status. However, as tachycardia is not specific for shock, assess other clinical parameters also. Specifically, guidelines recommend aiming for the following:31
- CRT ≤ 2 seconds;
- Normal blood pressure for age;
- Normal pulses, equal peripheral and centrally;
- Warm extremities;
- Urine output of 1 mL/kg/hr;
- Normal mental status;
- Euglycemia;
- Normal ionized calcium.
As for the blood pressure, albeit a useful indicator of shock and macrovascular circulation, when all other clinical parameters are reassuring and improving, some authors recommend against its use as an isolated marker of persistent shock state in children to guide further aggressive therapy.13
Despite a lack of evidence for the value of ultrasound as a tool for assessing the intravascular volume in the pediatric population, its role is very important in the adult requiring fluid resuscitation.75,76 Further studies are required in children.
Invasive Methods: Central Venous Pressure and Central Venous Oxygen Saturation. Central venous pressure (CVP) monitoring is one of the most commonly used methods for early goal-directed therapy. The target CVP recommended is 8 to 12 mmHg in patients with spontaneous breathing, and 12 to 15 mmHg in those who are receiving positive pressure ventilation.57 However, despite the wide use of CVP to guide fluid therapy, caution is recommended with its use as an isolated parameter because a myriad of other factors (diastolic dysfunction, pulmonary hypertension, or increased intrathoracic pressure) can affect it.77
In children, current recommendations are to monitor the central venous oxygen saturation (SCVO2) during resuscitation, aiming for a saturation ≥ 70%.31 de Oliviera et al showed that targeting an SCVO2 > 70% was associated with decreased mortality from 39.2% to 11.8% (P = 0.002) in pediatric septic shock.78 However, other studies have failed to prove the benefit of SCVO2 monitoring over less-invasive strategies, such as lactate serial checks, in terms of predicting in-hospital mortality.79 SCVO2 can be measured either with a catheter tip in the superior vena cava or from a femoral
catheter with its tip in the inferior vena cava.
Disposition
Admit all children with proven or suspected septic shock for observation. If the hemodynamic abnormalities (e.g., tachycardia and poor perfusion) were reversed in the emergency department, the child should be admitted to the inpatient floor in collaboration with PICU. All children with septic shock should be admitted to a PICU. As soon as possible after recognizing septic shock, inform the PICU team or initiate transfer to a specialized, probably tertiary care, center with a PICU. This should not delay any ED resuscitative measures and care, but will allow the child to reach definitive care faster.
Summary
Pediatric septic shock is a high-stakes diagnosis with elevated morbidity and mortality if not recognized and treated appropriately. As in adults, providers should attempt to recognize it early; ED trigger tools will help. Develop local ED pathways to treat rapidly and appropriately while closely monitoring response to treatment. Children may require up to 60 mL/kg of normal saline over one hour. Recognizing the suspected infectious etiology early will help with the choice of antibiotics; however, consider MRSA in all patients. Finally, if the child is not responding as expected, consider an alternative diagnosis.
Keep in mind the following common pitfalls:
- Missing early sepsis presenting solely as tachycardia with fever. Be sure to investigate any child who has persistent tachycardia.
- Not reassessing response to therapy and hence not escalating care as per the guidelines, or missing an alternative diagnosis.
REFERENCES
- Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14:686-693.
- Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013, Statistical Brief # 204, Health Care Costs and Utilization Project (HCUP) Statistical Briefs. May 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK368492/. Accessed Sept. 21, 2017.
- Nduka O, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin 2009;25:677-702.
- Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respi Crit Care Med 2015;191:1147-1157.
- Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: A multicenter retrospective cohort study. Lancet Infect Dis 2015;15:46-54.
- Novosad SA, Sapiano MR, Grigg C, et al. Vital Signs: Epidemiology of Sepsis: Prevalence of Health Care Factors and Opportunities for Prevention. MMWR Morb Mortal Wkly Rep 2016;65:864-869. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6533e1.htm. Accessed Sept. 18, 2017.
- Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2-8.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-810.
- Kawasaki T. Update on pediatric sepsis: A review. July 20, 2017. Available at: https://jintensivecare.biomedcentral.com/articles/10.1186/s40560-017-0240-1. Accessed Aug. 31, 2017.
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-1638.
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-377.
- Biban P, Gaffuri M, Spaggiari S, et al. Early recognition and management of septic shock in children. Pediatr Rep 2012;4:e13.
- Martin K, Weiss SL. Initial resuscitation and management of pediatric septic shock. Minerva Pediatr 2015;67:141-158.
- Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: Current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014;15:828-838.
- Pedro Tda C, Morcillo AM, Baracat EC. Etiology and prognostic factors of sepsis among children and adolescents admitted to the intensive care unit. Rev Bras Ter Intensiva 2015;27:240-246.
- Gaines NN, Patel B, Williams EA, Cruz AT. Etiologies of septic shock in a pediatric emergency department population. Pediatr Infect Dis J 2012;31:1203-1205.
- Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14:686-693.
- Chang TH, Wu ET, Lu CY, et al. Pathogens and outcomes in pediatric septic shock patients supported by extracorporeal membrane oxygenation. J Microbiol Immunol Infect 2017;pii: S1684-1182(17)30152-4. [Epub ahead of print].
- Leung CH, Tseng HK, Wang WS, et al. Clinical characteristics of children and adults hospitalized for influenza virus infection. J Microbiol Immunol Infect 2014;47:518-525.
- Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics 2017;140(1). doi: 10.1542/peds.2017-0695. Epub 2017; Jun 6.
- Remick DG. Pathophysiology of sepsis. Am J Pathol 2007;170:1435-1444.
- NICE. Sepsis: Recognition, diagnosis and early management. In: NICE guideline [NG51]. July 2016. Updated Sept 2017. Available at: https://www.nice.org.uk/guidance/ng51. Accessed Sept. 21, 2017.
- Promerantz W, Weiss S. Systemic inflammatory response syndrome (SIRS) and sepsis in children: Definitions, epidemiology, clinical manifestations, and diagnosis. Torrey S, Kaplan S, Randolph GA, eds. UpToDate. Waltham, MA: UpToDate Inc. Available at: http://www.uptodate.com. Accessed Sept. 26, 2017.
- Butt W. Septic shock. Pediatr Clin North Am 2001;48:601-625.
- PALS Algorithms 2017. Available at: https://www.acls-pals-bls.com/algorithms/pals/#shock. Accessed Sept. 21, 2017.
- Guzman-Cottrill JA, Cheesebrough B, Nadel S, et al. Chapter 11. The systemic inflammatory response syndrome (SIRS), sepsis and septic shock. In: Principles and Practice of Pediatric Infectious Diseases. Part II: Clinical Syndromes and Cardinal Features of Infectious Diseases: Approach to Diagnosis and Initial Management. Section A: Septicemia, Toxin- and Inflammation-Mediated Syndromes. Philadelphia: Elsevier Ltd., Inc. 2012.
- Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med 2017;45:1061-1093.
- Faustino EV, Bogue CW. Relationship between hypoglycemia and mortality in critically ill children. Pediatr Crit Care Med 2010;11:690-698.
- Wintergerst KA, Buckingham B, Gandrud L, et al. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics 2006;118:173-179.
- Branco RG, Garcia PC, Piva JP, et al. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med 2005;6:470-472.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637.
- Guignard JP, Santos F. Laboratory investigations. In: Avner ED, Harmon WE, Niaudet P, eds. Pediatric Nephrology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2004: 404.
- Cronan K, Kost SI. Renal and Electrolyte Emergencies. In: Fleisher G, Ludwig S, Henretig FM, eds. Pediatric Emergency Medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006: 873.
- Sawaya RD. Fluid and electrolyte management: Part 2: Electrolyte disturbances and acid-base disorders. Pediatr Emerg Med Rep 2016;21:49-64.
- Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011;128:595-610.
- Parmar A, Langenberg C, Wan L, et al. Epidemiology of septic acute kidney injury. Curr Drug Targets 2009;10:1169-1178.
- Majumdar A. Sepsis-induced acute kidney injury. Ind J Crit Care Med 2010;14:14-21.
- Yildizdas D, Yapicioglu H, Yilmaz H, Sertdemir Y. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child 2004;89:176-180.
- Batra P, Dwivedi AK, Thakur N. Bedside ABG, electrolytes, lactate and procalcitonin in emergency pediatrics. Int J Crit Ill Inj Sci 2014;4:247-252.
- Randolph AG, McCulloh RJ. Pediatric sepsis: Important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence 2014;5:179-189.
- Engorn B, Flerlage J. Chapter 27: Blood Chemistries and Body Fluids. In: Engorn B, Flerlage J, eds. The Harriet Lane Handbook. 20th ed. Philadelphia: Elsevier Saunders; 2014: 639-658.
- Scott HF, Donoghue AJ, Gaieski DF, et al. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med 2012;19:1276-1280.
- Bai Z, Zhu X, Li M, et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr 2014;14:83.
- Choudhary R, Sitaraman S, Choudhary A. Lactate clearance as the predictor of outcome in pediatric septic shock. J Emerg Trauma Shock 2017;10:55-59.
- Scott HF, Brou L, Deakyne SJ, et al. Lactate clearance and normalization and prolonged organ dysfunction in pediatric sepsis. J Pediatr 2016;170:149-155.e1-4.
- Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: An individual patient data meta-analysis. Clin Infect Dis 2012;55:651-662.
- Matthaiou DK, Ntani G, Kontogiorgi M, et al. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med 2012;38:940-949.
- Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: A systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:v-xxv, 1-236..
- Jacobs DM, Holsen M, Chen S, et al. Procalcitonin to detect bacterial infections in critically ill pediatric patients. Clin Pediatr 2017;56:821-827.
- Lautz AJ, Dziorny AC, Denson AR, et al. Value of procalcitonin measurement for early evidence of severe bacterial infections in the pediatric intensive care unit. J Pediatr 2016;179:74-81.e2.
- Casado-Flores J, Blanco-Quiros A, Asensio J, et al. Serum procalcitonin in children with suspected sepsis: A comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med 2003;4:190-195.
- Murphy CG, Van de Pol AC, Harper MB, Bachur RG. Clinical predictors of occult pneumonia in the febrile child. Acad Emerg Med 2007;14:243-249.
- Foo CPZ, Sangha G, Seabrook J, Foster J. Systemic inflammatory response in the pediatric emergency department: A common phenomenon that does not predict severe illness. Crit Care 2014;18(Suppl 2):P38.
- Waltzman M. Initial evaluation of shock in children. Torrey S, ed. UpToDate. Waltham, MA: UpToDate Inc. Available at: http://www.uptodate.com. Accessed July 14, 2017.
- Oliveira CF, Noqueira de Sa FR, Oliverira DS, et al. Time and fluid sensitive resuscitation for hemodynamic support of children in septic shock: Barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care 2008;24:810-815.
- Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 2003;112:793-799.
- Lane RD, Funai T, Reeder R, Larsen GY. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics 2016;138: pii: e20154153. Epub 2016 Sep 7.
- PRISM Investigators; Rowan KM, Angus DC, Bailey M, et al. Early, goal-directed therapy for septic shock — A patient-level meta-analysis. N Engl J Med 2017;376:2223-2234.
- Rose EC. The evidence-based use of intraosseous lines in pediatric patients. Pediatr Emerg Med Pract 2012;9.
- Weiss SL, Keele L, Balamuth F, et al. Crystalloid fluid choice and clinical outcomes in pediatric sepsis: A matched retrospective cohort study. J Pediatr 2017;182:304-310.
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483-2495
- Carlton KKL, Engorn B, Flerlage J. Chapter 29: Drug Dosages. In: Engorn B, Flerlage J, eds. The Harriet Lane Handbook. 20th edition. Philadelphia: Elsevier Saunders; 2014: 645-698.
- Lampin ME, Rousseaux J, Botte A, et al. Noradrenaline use for septic shock in children: Doses, routes of administration and complications. Acta Paediatr 2012;101:426-430.
- Tourneux P, Rakza T, Abazine A, et al. Noradrenaline for management of septic shock refractory to fluid loading and dopamine or dobutamine in full-term newborn infants. Acta Paediatr 2008;97:177-180.
- Ramaswamy KN, Singhi S, Jayashree M, et al. Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med 2016;17:502-512.
- Ventura AM, Shieh HH, Bousso A, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med 2015;43:2292-2302.
- Menon K, McNally D, Choong K, Sampson M. A systematic review and meta-analysis on the effect of steroids in pediatric shock. Pediatr Crit Care Med 2013;14:474-480.
- Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42:2409-2417.
- Sterling SA, Miller WR, Pryor J, et al. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: A systematic review and meta-analysis. Crit Care Med 2015;43:1907-1915.
- Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017;376:2235-2244.
- Chong SL, Ong GY, Venkataraman A, et al. The golden hours in paediatric septic shock — current updates and recommendations. Ann Acad Med Singapore 2014;43:267-274.
- Franco K. Guidelines for the management of pediatric severe sepsis and septic shock. Guidelines Update 2013;5:9.
- Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: A meta-analysis. Crit Care Med 2012;40:2945-2953.
- Carcillo JA, Kuch BA, Han YY, et al. Mortality and functional morbidity after use of PALS/APLS by community physicians. Pediatrics 2009;124:500-508.
- Karacabey S, Sanri E, Guneysel O. A non-invasive method for assessment of intravascular fluid status: Inferior vena cava diameters and collapsibility index. Pak J Med Sci 2016;32:836-840.
- Ilyas A, Ishtiaq W, Assad S, et al. Correlation of IVC diameter and collapsibility index with central venous pressure in the assessment of intravascular volume in critically ill patients. Cureus 2017;9:e1025.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013;41:1774-1781.
- de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: An outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med 2008;34:1065-1075.
- Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009;32:35-39.
Pediatric sepsis is a high-stakes diagnosis that requires vigilance to make an early, timely diagnosis. Aggressive resuscitation, including fluids, antibiotics, and vasoactive agents, may be necessary. Rapidly changing standard of care also makes sepsis a critical diagnosis for clinicians.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
