Drug Criteria & Outcomes: Urokinase (Abbokinase) formulary evaluation
Drug Criteria & Outcomes: Urokinase (Abbokinase) formulary evaluation
By Sherelia Duncan, PharmD candidate
Harrison School of Pharmacy
Auburn (AL) University
Written while on clinical rotation at Huntsville (AL) Hospital
Introduction
Due to manufacturing problems, the U.S. Food and Drug Administration (FDA) took urokinase (Abbokinase) off the market in December 1998. During inspection of the manufacturing facility in October and November of 1998, urokinase production was found to differ from Good Manufacturing Practice regulations. The FDA suggested that the manufacturer, Abbott Laboratories, not release any of the product until the FDA reviewed the inspection findings. The FDA issued a warning in January 1999 stating that products such as urokinase, which is made from human source materials, have the potential to transmit infectious agents. Even though Abbott had procedures in place to reduce such risks, the FDA inspection revealed deficiencies in certain procedures.
Since then, Abbott has corrected its manufacturing problems, and urokinase recently returned to the market. Its place in therapy is now being re-evaluated. This evaluation will focus on thrombolytic use in peripheral arterial occlusion and pulmonary embolism.
Thrombolytics compared
• Urokinase — an enzyme produced by the kidneys and excreted in urine
• Alteplase — a recombinant DNA form of the enzyme human-tissue-type plasminogen activator
• Reteplase — a recombinant DNA form of the enzyme human-tissue-type plasminogen activator
Background/mechanism of action
When a vessel is occluded, the body signals the release of tissue plasminogen activator (t-PA) from endothelial cells. Plasminogen is an inactive precursor that is converted to plasmin. Plasmin, an enzyme, dissolves intravascular fibrin clots and other plasma proteins, including several coagulation factors.
T-PA binds to fibrin and converts plasminogen (which also is bound to fibrin) to plasmin. T-PA has little effect on circulating plasminogen because it is rapidly cleared from blood or inhibited by circulating inhibitors, plasminogen activator inhibitor-1, and plasminogen activator inhibitor-2. Alpha2-antiplasmin inactivates plasmin by forming a stable complex with it. In healthy people, the fibrinolytic system is in balance such that unwanted fibrin thrombi are removed, while fibrin in wounds persists.
Thrombolytics act like tissue plasminogen activators, converting plasminogen to plasmin. Alpha2-antiplasmin plasma concentrations are sufficient to inhibit about 50% of plasmin activity. However, when thrombolytics are used, massive activation of plasminogen occurs and the inhibitor is overwhelmed, so much so that free plasmin causes a systemic lytic state. Thrombolytic drugs dissolve both pathological thrombi and fibrin deposits at sites of vascular injury.
Urokinase is slightly different from alteplase and reteplase because it directly activates plasminogen to plasmin. Urokinase can bind to plasminogen directly; however, alteplase and reteplase activate plasminogen that is bound to fibrin. Plasminogen is present in thrombi and emboli; therefore, activation by urokinase occurs within, as well as on the surface of, thrombi and emboli.
Alteplase and reteplase have similar mechanisms of action. Both preferentially activate plasminogen that is bound to fibrin, which theoretically confines fibrinolysis to the formed thrombus and avoids systemic activation.
Clinical uses (*FDA-labeled use)
Urokinase
• peripheral arterial occlusion
• acute pulmonary embolism*
• acute myocardial infarction
• catheter occlusion
Alteplase
• peripheral arterial occlusion
• acute pulmonary embolism *
• acute myocardial infarction*
• acute ischemic stroke*
• catheter occlusion
Reteplase
• peripheral arterial occlusion
• pulmonary embolism
• acute myocardial infarction*
Dosing of urokinase — adults
• Peripheral arterial occlusion
— The most common initial dose is 240,000 international units/hour (IU/hr) for two to four hours or until blood flow is restored.
— The most common maintenance regimens are: 1) 120,000 IU/hr to a maximum of 48 hours or 2) 120,000 IU/hr for two hours, then 60,000 IU/hr until lysis is complete. The dose is administered intra-arterially in close proximity to the thrombus or intra-thrombus (directly in the thrombus). The intravenous route is not used.
Several different regimens have been used in studies, including:
— A bolus dose of 60,000 IU administered into the thrombus, followed by 240,000 IU/hr for two hours, 120,000 IU/hr for two hours, and 60,000 IU/hr for up to a maximum of 20 hours.
— An initial dose of 150,000 IU over one-half to two hours, followed by continuous infusion of 50,000 IU/hr. The average time to clot lysis was 26 hours.
— An initial dose of 240,000 IU/hr until blood flow was restored, followed by a maintenance dose of 60,000 IU/hr or 120,000 IU/hr until clot lysis was achieved.
• Pulmonary embolism
— A loading dose of 4,400 IU/kg of urokinase is administered over a period of 10 minutes, followed by continuous infusion of 4,400 IU/kg/hr of urokinase for 12 hours. No other medications should be added to the urokinase solution.
• Acute myocardial infarction
— The optimal dose of urokinase for intracoronary thrombolysis is 6,000 IU/min for up to two hours.
• Catheter clearance
— The recommended dose is 5,000 IU × one dose intra-catheter (IC).
— A second injection of urokinase may be necessary.
Dosing of urokinase — children
Urokinase is not FDA-approved for children. The safety and efficacy of urokinase therapy has not been established in pediatric patients.
• Catheter clearance
— The recommended dose is 5,000 IU × one dose IC.
— A second injection of urokinase may be necessary.
• Central thrombosis
Urokinase was used in neonates to treat four cases of central thrombosis via umbilical artery catheter into the abdominal aorta. The dose used was a 4,400 IU/kg loading dose followed by a 4,000 to 20,000 IU/kg/hr maintenance dose. The duration of therapy ranged from three to nine days.
Dosing of alteplase — adults
• Peripheral arterial occlusion
The literature provides data on weight-based and non-weight-based dosing regimens ranging from 0.02 to 0.1 mg/kg/hr and 0.25 to 10 mg/hr. The risk of bleeding is increased with higher doses. In one study, for example, patients received a 10 mg bolus, followed by 5 mg/hr for up to 24 hours. In this study, the t-PA patients experienced more major bleeding episodes than the urokinase group; however, both drugs demonstrated similar efficacy. In another study, patients received an average dose of 0.86 ± 0.5 mg/hr. This study used the lower dosage range and found that urokinase and alteplase had similar safety and efficacy. Thus, in studies in which the patients received doses of t-PA > 2.0 mg/hr, there was a greater incidence of bleeding in the t-PA group than in the studies that gave doses of < 2.0 mg/hr. The Society of Cardiovascular and Interventional Radiology (SCVIR) dosing guidelines recommend the following doses:
— The dose is 0.001-0.02 mg/kg/hr (weight-based) or 0.12-2.0 mg/hr (non-weight-based); dose should not exceed 2 mg/hr.
— The total dose is < 40 mg for catheter-directed therapy. A single bolus dose should not exceed 10 mg.
— The dose of concurrent heparin therapy is 2,500 units (U) bolus followed by 500 U/hr. Activated partial thromboplastin time (aPTT) should be monitored.
• Acute pulmonary embolism
The dose is 100 mg over two hours.
• Acute myocardial infarction, front-loading dose (weight-based)
— For patients who weigh more than 67 kg, the total dose is 100 mg over 1.5 hours; infuse 15 mg over one to two minutes. Infuse 50 mg over 30 minutes. Begin heparin 5,000-10,000 U bolus followed by continuous infusion of 1,000 U/hr. Infuse the remaining 35 mg of alteplase over the next hour.
— For patients who weigh less than 67 kg, the total dose is 1.25 mg/kg; infuse 15 mg IV bolus over one to two minutes. Then infuse 0.75 mg/kg (not to exceed 50 mg) over the next 30 minutes, followed by 0.5 mg/kg over the next 60 minutes (not to exceed 35 mg). Begin heparin 5,000-10,000 U bolus followed by continuous infusion of 1,000 U/hr.
• Acute ischemic stroke
The dose should be given within the first three hours of the onset of symptoms. The loading dose is 0.09 mg/kg as a bolus, followed by 0.81 mg/kg as a continuous infusion over 60 minutes. The maximum total dose should not exceed 90 mg.
• Central venous catheter occlusion
For patients who weigh 10-30 kg, the dose is 2 mg (1 mg/1 mL) instilled into the occluded catheter. The solution should be allowed to stay in the catheter for 30 minutes. Then catheter function should be assessed by attempting to aspirate blood and catheter contents. If catheter function is not restored, allow the solution to stay in the catheter for 90 additional minutes (120 minutes total). The catheter’s functioning should be reassessed after a total of 120 minutes. If catheter function restoration fails, this entire process may be repeated one additional time.
Dosing of alteplase — children
• For pediatric patients older than 2 years of age and weighing 10-29 kg, the dose to be given is 1 mg/1 mL (max dose of 2 mg/2 ml.)
• Pediatric patients older than 2 years of age and weighing 30 kg or more should receive 2 mg/ 2 mL.
• Alteplase has not been studied in children younger than 2 years of age or weighing less than 10 kg.
Dosing of reteplase — adults
• Acute myocardial infarction
— Reteplase is administered as two 10-unit bolus injections each over two minutes. The second dose should be given 30 minutes after initiation of the first injection.
— Reteplase should be given through a line in which no other medications are being administered. Reteplase is incompatible with heparin; therefore, if both drugs are being given to the patient, the line should be flushed before and after reteplase with either 0.9% sodium chloride or 5% dextrose solution.
• Pulmonary embolism (not FDA-approved)
Administer 10 U IV over two minutes, wait 30 minutes, and then repeat the dose.
Dosing of reteplase — children
Reteplase is not recommended in children.
Pharmacokinetics
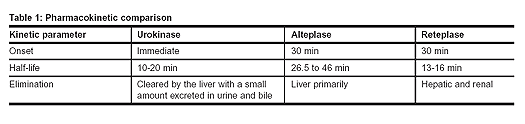
A comparison of the pharmacokinetic parameters for urokinase, alteplase, and reteplase is presented in Table 1.
Monitoring parameters
• Before therapy begins, baseline levels of hematocrit, platelet count, thrombin time, aPTT, prothrombin time (PT), or fibrinogen should be evaluated.
• To avoid dislodgement of a deep-vein thrombosis, do not take lower-extremity blood pressure.
• During the infusion, a decrease in plasminogen and fibrinogen (indicating a prolongation in clotting time of coagulation tests) and an increase in fibrinogen degradation products (FDP) generally confirm a lytic state. Therefore, a lytic state can be confirmed by checking fibrinogen (200-400 mg/dL), aPTT (22.5-38.7 seconds), and PT (10.9-12.2 seconds) approximately four hours after starting therapy.
• During therapy, the following should be monitored every eight to 12 hours: CBC, reticulocyte count, platelet count, DIC panel (fibrinogen, plasminogen, FDP, D-dimer, PT, PTT), and thrombosis panel (AT-III, protein C).
• During therapy, the patient should be monitored for signs of bleeding, such as hematuria, gastrointestinal bleeding, gingival bleeding, and stool guaiac.
Contraindications/warnings
Contraindications for thrombolytic therapy are relative, not absolute. Therefore, the benefits and risks of this therapy should be evaluated for each patient. Thrombolytics are contraindicated in the following situations:
• hypersensitivity to a thrombolytic product;
• active internal bleeding;
• surgery or trauma within 10 days;
• intracranial neoplasm;
• arteriovenous malformation, or aneurysm;
• severe uncontrolled hypertension (greater than 180/110 mmHg);
• recent stroke, serious head trauma, or intracranial/intraspinal surgery; and
• pregnancy.
Warnings
• If alteplase is administered three to four hours after a major ischemic stroke, it may cause cerebral edema with fatal brain herniation. Therefore, it is very important that alteplase is administered within three hours of symptom onset.
• If a serious bleed at a critical location (intracranial, gastrointestinal, retroperitoneal, pericardial) occurs, immediately discontinue the thrombolytic and heparin.
Adverse drug reactions
• Urokinase
— More than 10%: Bleeding, arrhythmias, hypotension, periorbital swelling, and dyspnea.
— Less than 1%: Anaphylaxis, bronchospasm, chills, nausea, vomiting, rash, epistaxis, and anemia.
• Alteplase
— 1-10%: Bleeding, hypotension, fever, ecchymosis, and nausea and vomiting.
— Less than 1%: Epistaxis, gingival hemorrhage, intracranial hemorrhage, pericardial hemorrhage, retroperitoneal hemorrhage, and allergic reactions.
• Reteplase
— More than 10%: Bleeding.
— Less than 1%: Allergic reactions, intracranial hemorrhage, and cholesterol embolization.
Drug interactions
As expected, all three of these drugs interact with other drugs that potentiate the risk of bleeding, such as anticoagulants, antiplatelet agents (acetylsalicylic acid, nonsteroidal anti-inflammatory drugs, dipyridamole, and GP IIb/IIIa inhibitors), low-molecular-weight heparins, and other thrombolytics.
Alteplase has an additional drug-drug interaction with nitroglycerin. Nitroglycerin increases hepatic blood flow, which may decrease alteplase plasma concentrations. This may decrease the efficacy of alteplase and increase the risk of reocclusion of the artery. Concomitant use of alteplase and nitroglycerin should be avoided if possible. If both drugs are necessary, the lowest effective dose of nitroglycerin should be used.
Ways to decrease potential medication errors with thrombolytics
There is a high risk for medication errors with the thrombolytics because each of the agents has a different dosing regimen, complex reconstitution instructions, and specific administration criteria. The following measures may prevent some of these errors:
• multidisciplinary education programs;
• education on differences in dosing;
• stressing that urokinase and reteplase are dosed in units and alteplase is dosed in milligrams;
• education on reconstitution procedures;
• stressing that these agents often are administered as a bolus dose followed by continuous infusion; and
• use of approved protocols.
Clinical trials
Meyerovitz MF, Goldhaber SZ, Reagan K, et al. Recombinant tissue-type plasminogen activator versus urokinase in peripheral arterial and graft occlusions: A randomized trial. Radiology 1990; 175:75-78.
Objective: To compare intra-arterial administration of recombinant-human-tissue-type plasminogen activator (rt-PA) with urokinase in patients with peripheral arterial or bypass graft occlusions.
Study design: A prospective randomized trial of 32 patients with peripheral arterial or bypass graft occlusions. Sixteen patients were randomized to receive rt-PA, and 16 to receive urokinase.
Inclusion criteria: Men or women 18 years of age and older who have a peripheral arterial or bypass graft occlusion as diagnosed by an arteriograph.
Exclusion criteria:
• Active internal bleeding within three weeks
• Recent (within one year) cerebrovascular accident, intracranial or intraspinal surgery, or intracranial neoplasm
• Internal surgery or organ biopsy within 10 days
• Open-heart surgery within three weeks
• "One plus" or more positive occult blood at stool exam
• Severe impairment of hepatic function
• Severe uncontrolled arterial hypertension or diabetic hemorrhagic retinopathy
• Pregnancy or lactation
Endpoints:
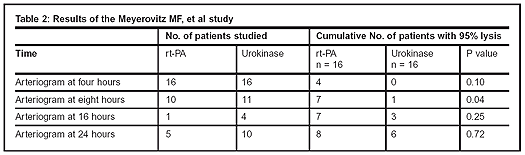
• Arteriograms were taken at baseline and at four, eight or 16, and 24 hours. The endpoint was 95% or greater clot lysis.
Treatment regimens:
• The rt-PA dose was given as a 10 mg bolus administered into the thrombus, followed by 5 mg/hr for up to 24 hours.
• The urokinase dose was given as a 60,000 IU bolus administered into the thrombus, followed by 240,000 IU/hr for two hours, 120,000 IU/hr for two hours, and 60,000 IU/hr for up to a maximum of 20 hours.
• Also, all patients received concomitant IV heparin (3,000-5,000 U loading dose followed by 600-1,000 U/hr).
Results: Lysis occurred more rapidly for the rt-PA group at eight hours (P = 0.04). Five rt-PA patients and two urokinase patients experienced major bleeding episodes (P = 0.39). Fibrinogen levels were similar in both groups at baseline (P = 0.79), four hours (P = 0.59), eight hours (P = 0.34), and 16 hours (P = 0.95). However, fibrinogen levels were significantly lower in the rt-PA group than in the urokinase group at 24 hours (P = 0.01).
After completing the study protocol of up to 24 hours of therapy, six urokinase patients and two rt-PA patients received additional therapy in an effort to successfully lyse their clots. The six urokinase patients received an additional 18-72 hours of therapy. Four of these patients achieved successful thrombolysis. The two rt-PA patients did not have successful lysis of their clots after an additional 1-36 hours of therapy.
There was no apparent difference in 30-day clinical success. Additional results of the study are summarized in Table 2.
Conclusions: The authors concluded that rt- PA and urokinase have similar efficacy at 24 hours of treatment. However, rt-PA did tend to cause more rapid lysis than urokinase. This study also demonstrated that rt-PA tended to cause more bleeding complications than urokinase, although this finding was not statistically significant. Overall, the authors concluded that rt-PA and urokinase yielded similar clinical outcomes.
Limitations and strengths: This unblinded study evaluated a small sample of patients and did not clearly define the inclusion criteria. However, the study was randomized and approved by the Institutional Review Board and the FDA. In addition, written informed consent was obtained from all patients, and baseline characteristics were similar.
Cina CS, Goh R, Chan J, et al. Intra-arterial catheter-directed thrombolysis: Urokinase versus tissue plasminogen activator. Ann Vasc Surg 1999; 13:571-575.
Objective: To evaluate whether t-PA and urokinase have similar efficacy and safety in the management of ischemic limbs.
Study design: This prospective cohort study compared catheter-directed fibrinolysis with urokinase and t-PA in the management of limb ischemia. Thirty-four patients received urokinase; 24 patients received t-PA.
Inclusion criteria: Patients with non-embolic limb ischemia of less than three months’ duration caused by occlusion of native arteries or bypass grafts.
Exclusion criteria: Exclusion criteria were not included.
Endpoints:
• Primary: Angiographic recanalization of the occluded vessel.
• Secondary: Duration of the procedure.
Treatment regimens:
• The 150,000 IU bolus dose of urokinase was given over one-half to two hours, followed by continuous infusion of 50,000 IU/hr.
• The 5 mg bolus dose of t-PA was followed by an infusion of 1 mg/hr.
• Also, concomitant heparin was administered at 400 U/hr.
Results:
• Primary endpoint — T-PA: 18 of 24 patients had successful recanalizations.
• Primary endpoint — Urokinase: 27 of 34 patients had successful recanalizations.
• Secondary endpoint: Recanalization was established at an average time of 14.9 hr in the t-PA group and 25.5 hr in the urokinase group (P = 0.009).
• The lowest mean fibrinogen level was not statistically different between the groups (P = 0.3). Also, the risk of death was not significantly different between the groups (P = 0.29).
• There were significantly more major bleeds in the t-PA group than in the urokinase group. Major bleeds occurred in 11 t-PA patients and three urokinase patients (P = 0.0018).
Conclusions: The authors concluded that t-PA and urokinase have similar overall efficacy; however, time to lysis was shorter with t-PA. This study also demonstrated that t-PA had a higher incidence of bleeding complications.
Limitations and strengths: Although the baseline characteristics of this cohort study were similar, inclusion and exclusion criteria were not clearly defined and the sample size was small.
Sugimoto K, Hofmann LV, Razavi MK, et al. The safety, efficacy and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg 2003;37:512-517.
Objective: To compare the efficacy, complications, and costs associated with low-dose (less than 2 mg/hr) alteplase vs. urokinase for catheter-directed treatment of acute peripheral arterial occlusive disease and deep-vein thrombosis.
Study design: This retrospective review evaluated 89 patients with 93 involved limbs that were treated with either t-PA with subtherapeutic heparin or urokinase with full heparin at a single center.
Inclusion criteria:
• Acute limb ischemia (less than 14 days)
• Acute symptomatic deep-vein thrombosis (up through 30 days)
Exclusion criteria: Exclusion criteria were not defined.
Endpoints: Success rates, complications, infusion time, and costs were included as endpoints.
Treatment regimen:
• The urokinase dose was typically 120,000 IU/ hr (range: 50,000-240,000 IU/hr). Full-dose heparin was given to maintain the partial thromboplastin time at 1.5-2 times the baseline.
• The t-PA dose was typically 0.5 mg/hr for peripheral arterial occlusive disease and 1.0 mg/hr for deep-vein thrombosis (range: 0.25-2.0 mg/hr). Subtherapeutic heparin was administered to maintain the partial thromboplastin time at less than 1.5 times the baseline.
Results: Results of the Sugimoto et al study are presented in Table 3.
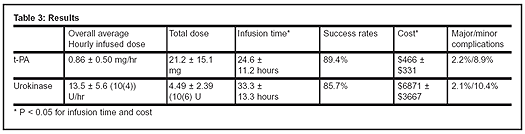
Conclusions: The authors concluded that low-dose t-PA with sub-therapeutic heparin and urokinase with full-dose heparin are similar in safety and efficacy. However, t-PA was found to act significantly faster and was less expensive than urokinase.
Limitations: This retrospective review was neither blinded nor randomized and included a small sample size.
Studies comparing urokinase and alteplase
Recently, urokinase returned to the market, and its place in therapy must be re-established. Eleven trials were identified that have compared urokinase and alteplase for use in peripheral arterial occlusive disease. Most of these trials were small and not blinded. The majority of these trials demonstrated that urokinase and alteplase have similar efficacy and safety for the treatment of peripheral arterial occlusive disease. A number of trials have demonstrated similar rates of bleeding for both urokinase and alteplase. Some studies found that alteplase had a greater risk of bleeding than urokinase; however, these trials are small, and, in some cases, this finding could be due to the use of larger doses of alteplase. One trial reported more bleeding complications with urokinase than with alteplase; however, the statistical significance was not stated. Most of the studies found that alteplase acts more rapidly than urokinase.
Three trials evaluated the cost of thrombolytics, and all three found that alteplase is less expensive than urokinase. Two of the trials also evaluated length of stay and found that alteplase decreased length of stay and therefore decreased cost. Most of the trials comparing urokinase and alteplase have several limitations, including small sample size, lack of blinding and randomization, and vague inclusion and exclusion criteria.
The FDA has approved urokinase and alteplase for use in acute pulmonary embolism. Three randomized clinical trials have compared t-PA and urokinase for pulmonary embolism. All three of these trials demonstrated similar safety and efficacy between these two agents for pulmonary embolism. These studies also found that alteplase acts more rapidly; however, one study demonstrated that a novel dosing regimen of urokinase (3 million IU/2 hrs, with the initial 1 million IU given as a bolus over 10 minutes) resulted in no significant difference in lysis at two hours vs. alteplase. As in the studies conducted to evaluate peripheral arterial occlusive disease, these studies have several limitations, including small sample size and vague inclusion and exclusion criteria. To date, no large randomized blinded clinical trials exist to compare available thrombolytics for peripheral arterial occlusive disease or pulmonary embolism.
Conclusion
It is difficult to draw definite conclusions about the differences in safety and efficacy of alteplase and urokinase because the comparative trials are so dissimilar. The trials are hard to compare because they have inconsistent study designs, various dosing regimens and administration techniques, and different safety and efficacy endpoints. Nonetheless, the literature that is available appears to demonstrate similar efficacy and safety between low-dose alteplase and urokinase for peripheral arterial occlusive disease and pulmonary embolism. In the future, it would be beneficial to have larger randomized blinded trials to lend more support to this conclusion.
As for reteplase, at this time there are few studies available evaluating its use for peripheral arterial occlusion, and no literature is available for its use in the treatment of pulmonary embolism. One retrospective cohort study found that reteplase had a satisfactory efficacy and safety profile in the treatment of peripheral arterial occlusive disease. However, controlled clinical trials are needed to compare different regimens of reteplase and to compare reteplase to different thrombolytics.
Based on the available data, alteplase appears to be the best initial choice for treatment of peripheral arterial occlusive disease and pulmonary embolism, due to the advantages of more rapid lysis and decreased expense. If the maximum dose of alteplase recommended by the SCVIR for peripheral arterial occlusive disease fails, then urokinase is a viable alternative. Urokinase also is an alternative agent for pulmonary embolism.
Resources
• Alteplase. In: McEvoy GK, ed. AHFS 2002: Drug Information. Bethesda, MD: American Society of Health System Pharmacists; 2002:1525-1535.
• Alteplase (monograph in electronic version). MICROMEDEX Healthcare Series. Englewood, CO: Micromedex; 2003.
• Alteplase. Drug Facts and Comparisons. St. Louis: Facts and Comparisons 2003:183-185.
• Baker WF. Thrombolytic therapy: Clinical applications. Hematol Oncol Clin North Am 2003;17:283-311.
• Cina CS, Goh R, Chan J, et al. Intraarterial catheter-directed thrombolysis: Urokinase versus tissue plasminogen activator. Ann Vasc Surg 1999;13:571-575.
• Dalen JE, Alpert JS, Hirsh J. Thrombolytic therapy for pulmonary embolism: Is it effective? Is it safe? When is it indicated? Arch Intern Med 1997;157:2550-2556.
• Goldhaber SZ, Kessler CM, Heit JA, et al. Recombinant tissue-type plasminogen activator versus a novel dosing regimen of urokinase in acute pulmonary embolism: A randomized controlled multicenter trial. J Am Coll Cardiol 1992; 20:24-30.
• Gilman AG, Limbird LE, Hardman JG, eds. Goodman’s & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw Hill; 2001.
• Kiproff PM, Yammine K, Potts J, et al. Reteplase in the treatment of acute lower extremity occlusions. J Thromb Thrombolysis 2002;13:75-79.
• Meyer G, Herve S, Charbonnier B, et al. Effects of intravenous urokinase versus alteplase on total pulmonary resistance in acute massive pulmonary embolism: A European multicenter double-blind trial. J Am Coll Cardiol 1992;19:239-245.
• Meyerovitz MF, Goldhaber SZ, Reagan K, et al. Recombinant tissue-type plasminogen activator versus urokinase in peripheral arterial and graft occlusions: A randomized trial. Radiology 1990;175:75-78.
• Peripheral Arterial Occlusion (PAO)/Activase and Urokinase in Peripheral Arterial Occlusion. Genentech. San Francisco; March 2003.
• Personal communication. Cindy Hall, Buyer, Pharmacy Department. Huntsville (AL) Hospital System, March 2003.
• Reteplase. In: McEvoy GK, ed. AHFS 2002: Drug Information. Bethesda, MD: American Society of Health System Pharmacists; 2002:1536-1537.
• Reteplase (monograph in electronic version). MICROMEDEX Healthcare Series. Englewood, CO: MICROMEDEX; 2003.
• Reteplase. Drug Facts and Comparisons. St. Louis: Facts and Comparisons 2003:186.
• Semba CP, Bakal CW, Clais KA, et al. Alteplase as an alternative to urokinase. J Vasc Interv Radiol 2000;11:279-287.
• Shortell CK, Queiroz R, Johansson M, et al. Safety and efficacy of limited-dose tissue plasminogen activator in acute vascular occlusion. J Vasc Surg 2001;34:854-859.
• Sugimoto K, Hofmann LV, Razavi MK, et al. The safety, efficacy and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg 2003;37:512-517.
• The STILE Investigators. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: The STILE trial. Ann Surg 1994;220:251-268.
• Urokinase. In: McEvoy GK, ed. AHFS 2002: Drug Information. Bethesda, MD: American Society of Health System Pharmacists; 2002:1545-1547.
• Urokinase (monograph in electronic version). MICROMEDEX Healthcare Series. Englewood, CO: MICROMEDEX; 2003.
• Urokinase. Drug Facts and Comparisons. St. Louis: Facts and Comparisons 2003:190.
• Vanscoy GJ, Rihn TL. "Peripheral Arterial Occlusion." In: Managing the Urokinase Shortage: Clinical and Economic Alternatives. Pittsburgh: University of Pittsburgh School of Pharmacy; 2001. Program No. 058-000-99-008-H01. Sponsored by grant from Genentech.
Due to manufacturing problems, the U.S. Food and Drug Administration (FDA) took urokinase (Abbokinase) off the market in December 1998. During inspection of the manufacturing facility in October and November of 1998, urokinase production was found to differ from Good Manufacturing Practice regulations.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
