Immigrant Medicine - The Emergency Department Perspective Part I: Evaluation, Diagnosis, and Treatment of Commonly Encountered Diseases
The Emergency Department Perspective Part I: Evaluation, Diagnosis, and Treatment of Commonly Encountered Diseases
Authors: Mary C. Meyer, MD, Attending Physician, Emergency Department, Kaiser Oakland and Kaiser Walnut Creek, Oakland, CA; Danica N. Barron, MD, Alameda County Medical Center, Highland General Hospital, Oakland, CA; R. Carter Clements, MD, FACEP, Assistant Chair, Department of Emergency Medicine, Alameda County Medical Center—Highland Campus, Oakland, CA; Clinical Instructor, Department of Internal Medicine, University of California, San Francisco.
Peer Reviewers: Judith C. Brillman, MD, FACEP, Associate Professor, Research Co-Director, Department of Emergency Medicine, University of New Mexico School of Medicine, Albuquerque; Hans House, MD, DTMU, Assistant Professor, Program in Emergency Medicine, University of Iowa, Iowa City.
The United States is a nation of immigrants. In the year 2000, it was estimated that 30 million immigrants resided in the United States,1,2 and 99% of the American population descended from immigrants.3 The past century has seen a large shift in the demographics of the United States immigrant population. From 1900 to1950, more than 90% of all immigrants arrived from Europe. In contrast, the number of Asian and Latin American immigrants increased dramatically in the latter half of the 20th century. U.S. Public Health Service statistics covering 1980-1984 show that 48% of arrivals came from Asia, 35% from Latin America, and 3% from Africa.1,4,5 A significant proportion of these were refugees and asylum-seekers, defined by the United Nations’ High Commission for Refugees as individuals who have left their home countries because of a "well-founded fear" of persecution as a result of race, religion, nationality, or political opinion.6,7 The United States population is becoming increasingly racially diverse due to immigration and higher birth rates among minority populations. Census data from the year 2000 revealed that 31% of United States residents were people of color (Hispanics and non-Hispanics who did not identify their race as White).2,8
Immigrants represent an epidemiologically unique population. They present with health problems that are particular to the non-United States-born, but at the same time are a heterogeneous group. Specific medical issues vary with the country of origin, legal status, and duration of stay in the United States. Further, certain subgroups, such as migrant workers from Latin America, frequently travel between their home country and the United States, thereby continually exposing themselves to medical maladies on both sides of the border.
As the number of foreign-born individuals in the United States continues to grow, the emergency physician (EP) must be adept at handling the medical issues unique to this population. Immigrants usually arrive in the United States with limited resources and little access to health care. Recent statistics note that 60% of low-income immigrants are uninsured, compared with 30% of low-income individuals born in the United States.9 Although most immigrants (85%) enter the United States legally, public misconceptions about immigrants’ legal status, role in the economy, and impact on the health system fuel anti-immigrant stereotypes and contribute to counterproductive public policy.2,10 During the past decade, the federal government and states have taken actions to limit immigrants’ access to health coverage and care. Most notably, the Personal Responsibility and Work Opportunity Reconciliation Act of 1996 affected cash assistance and the treatment of legal immigrants by increasing barriers to social welfare programs.2 Therefore, in the face of illness, many immigrants, both legal and illegal, have no option but to present to an emergency department (ED) for care.
The goal of this two-part series is to acquaint the EP with the symptoms, geographic distribution, and potential public health risks of diseases most prevalent in the immigrant population. Part I will review pertinent features of the history and physical examination, as well as commonly encountered disease processes with global distribution. Part II will focus on health problems that are localized to immigrants from specific regions of the world, including Latin America, Asia and South Asia, and Africa.—The Editor
Historical Evaluation
The initial evaluation of an immigrant patient begins with a thorough medical and travel history. Illness may reflect either an imported disease or a process acquired in the United States. Infections acquired in the United States may originate in other immigrants. In a recent case, 56 people acquired tuberculosis (TB) following exposure to a 9-year-old child from the Marshall Islands who was screened inadequately at the time of immigration.11
Length of Time in United States. Historical evaluation of the immigrant patient should begin by determining the length of time he or she has been living in the United States. As a general rule, certain patterns of illness can be predicted according to length of time after migration. In the first few years after arrival, health problems of immigrants tend to reflect the poverty and poor public health infrastructure of their countries of origin. During this period, the physician can expect to see diseases such as vaccine-preventable illnesses (i.e., measles, rubella), intestinal parasites, anemia, dental caries, and malnutrition. In general, cases of imported malaria usually will manifest within the first one to two years following arrival.12-14 For reasons poorly understood, the majority of reactivation TB typically is diagnosed within the first five years in the country.15-18
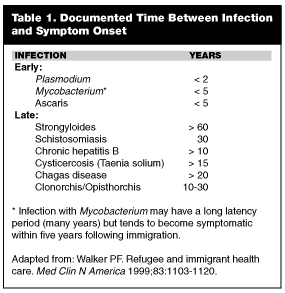
As time progresses, the immigrant’s overall health improves from nutrition and access to health care. At this point, a change in the pattern of illness is observed. Foreign-born individuals now present with the psychological effects of migration, such as post-traumatic stress disorder (PTSD), depression, and somatization. Imported infections still may occur but are more likely to be chronic and indolent, reflecting long latency periods. Table 1 lists some of the more common early and latent infections seen in the immigrant population.3 Malignancy, often stemming from infections acquired before migration, can present many years after arrival. Examples include hepatocellular cancer (secondary to hepatitis B) and bladder cancer (secondary to schistosomiasis). Rheumatic heart disease may be diagnosed incidentally during evaluation of an unrelated complaint.
A series of psychological stages, similar to the stages of death and dying, has been described in individuals who immigrate to a new country. As one immigrant author movingly wrote, "We have to live these half-lives of people who cannot forget what they used to be and who are afraid of being addressed in a foreign language, no longer able to utter anything meaningful."19 In 1979, Sluzki characterized five stages of migration in immigrants and refugees: 1) planning; 2) migration; 3) overcompensation; 4) decompensation; and 5) resolution.20 According to Sluzki, the overcompensation stage begins with arrival to the adopted country and is characterized by a survival mentality. The immigrant is operating in a "crisis mode" and only will address health problems that are urgent. The value of routine office visits or follow-up may be lost on the patient. After 1-2 years, this phase ends and the decompensation stage ensues, during which the impact of culture shock, loss, and mourning finally is felt. Somatization during this time period is common. The immigrant will begin to seek treatment for chronic health conditions that previously were ignored.20
Overseas Screening Exam. An equally important element of the history is the patient’s overseas screening exam. The U.S. Public Health Service requires that all immigrants and refugees who wish to establish permanent residence undergo medical screening, either in their home country or in the country of first asylum.21 Undocumented aliens, of course, do not undergo such screening. The primary purpose of the exam is to screen for infectious diseases of public health concern. This exam is neither comprehensive nor a guarantee of health but does include a history and physical, chest x-ray if older than 2 years, acid-fast sputum stain, HIV test, venereal disease research laboratory (VDRL), and screening for leprosy and mental disorders.3,7,21,22 Patients with "Class A" conditions (i.e., cholera, diphtheria, infectious TB, syphilis, plague, smallpox, yellow fever, HIV, and leprosy) are detained.3,7,21,22 Individuals with mental disorders are excluded only if they show evidence of harmful behavior.7,22 Recently revised guidelines allow people with chest x-rays consistent with active TB to enter the country provided they have negative sputum smears (i.e., noninfectious)7 and receive anti-TB therapy upon arrival. Of note, a sizable proportion of these patients are lost to follow-up after immigration, and it is not uncommon for them to arrive in an ED with active and untreated TB once symptoms have become more severe.
Inquiring about an individual’s screening exam can yield helpful information regarding the possibility of certain infectious diseases. For instance, patients may be able to describe a recent chest x-ray or report results of sexually transmitted disease (STD) screening. It should be stressed that undocumented aliens are at particularly high risk for imported infections and diseases. As a general rule, they fall into the lowest socioeconomic level and, because of their illegal status, have difficulty accessing medical care once in the United States. It is prudent to reassure patients that their legal status is confidential and it will not affect the care they receive.
Medical History. Along with a history of past medical problems, the physician should inquire specifically about immunization status and any experiences of trauma or torture. Survivors of torture can be expected to have high rates of both physical and somatic complaints.23-25 Medical pluralism (the use of both traditional and Western medicine) also is common in the immigrant population.26 Foreign-born individuals often use traditional healers and may have tried a variety of herbal medicines before seeking care in an ED. Finally, language and cultural barriers may present obstacles to both the history and physical exam. Asking patients questions about their notions of illness or symptoms may help decrease miscommunication.
Country of Origin. The country of origin is one of the most important elements of the history. Some disease processes are global, including TB, intestinal parasites, hepatitis B, and underimmunization. These entities are all diseases of poverty, malnutrition, and poor hygiene. Immigrants from a developing country are, by definition, at greater risk for such health problems. In contrast, other infections and diseases are localized to certain areas of the world. It is important to remember that some immigrants have spent years in multiple countries, living in refugee camps or detainment centers. Institutional living, such as an orphanage, poses different risks than living in a family home in the same country.11
Physical Examination
The physical examination of an immigrant should be directed toward finding manifestations of organic disease, signs of traditional health practices, and prior torture.
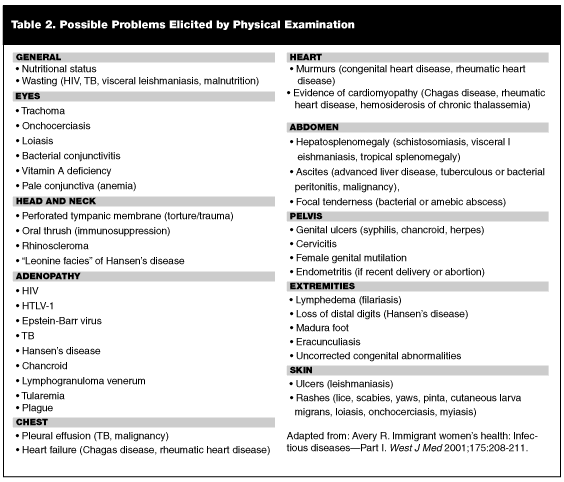
Organic Disease. Malnutrition is common among new arrivals; children, in particular, frequently demonstrate growth abnormalities.25 Other immigrants, especially from Latin America and Eastern Europe, are subject to obesity.25 Failure of children to display catch-up growth following immigration may unmask missed underlying disease, such as TB or anemia.11,26 Unresolving anemia following treatment may require testing for other conditions, including thalassemia trait and hemoglobinopathies. Toxin exposure, such as lead, environmental pollution, prenatal alcohol exposure, and radioactivity, should not be underestimated.11,27 Table 2 lists specific problems that may be elicited by physical examination.
Traditional Health Practices. Many immigrants continue to use traditional medicines and doctors after arrival in the United States. Coining and cupping, the practice of rubbing hot coins or cups over the skin to produce superficial burns, is common among the Southeast Asian population.7 Ritual scarification may be seen in individuals from Southeast Asia. Female circumcision, or female genital mutilation, occurs in some African societies and has been associated with multiple pelvic, urinary, and obstetric complications. Similarly, uvulectomy is performed as a treatment for sore throat in certain regions of Africa.26
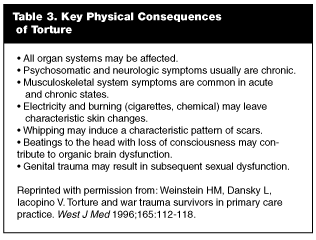
Prior Torture. It is estimated that 5-35% of the world’s refugees (1.1-8 million persons) have experienced torture.28 In 1975, the World Medical Association Declaration of Tokyo defined torture as, "the deliberate, systematic, or wanton infliction of physical or mental suffering by one or more persons, acting alone or on the orders of any authority, to force another person to yield information, to make a confession, or for any other reason."24 Immigrants who suffered torture in their home countries may have physical signs of the experience. (See Table 3). Scars can result from beatings, whippings, thermal or electrical burns, stretching, asphyxiation, and genital torture.7,24 Evidence of old fractures may be present. Falanga, torture that involves beating on the soles of the feet, can induce contractures and deformities.7 Other findings may include rectal bleeding, dysesthesia, plexopathy, radiculopathies, and STDs.24 It is crucial to remember that these injuries are a source of stress and embarrassment and must be approached carefully. Psychological torture, including sensory deprivation (isolation, darkness, constant noise); forced witnessing of beatings, rapes, or executions of friends and family; and restriction of physiological needs (food, water, sleep, and toilet) may have occurred alone or in concert with physical abuse.24 Of note, 20% of victims state that a physician was involved in their torture, which may contribute to a patient’s natural reluctance to discuss such experiences.29
Global Diseases Commonly Encountered in the Immigrant Population
Tuberculosis. Epidemiology. Once referred to as "the captain of all men of death,"30 TB is, without question, the most commonly encountered infection among United States immigrants. The World Health Organization (WHO) estimates that one-third of the world’s population is infected by Mycobacterium tuberculosis. In the year 2000, the Centers for Disease Control and Prevention (CDC) public health surveillance system noted that 46% of all TB cases in the United States occurred in immigrants, up from 27% in 1992.31 The majority of cases were concentrated in individuals from Central and South America, the Caribbean, and the Western Pacific region.31 Seven million foreign-born people with latent TB are estimated to reside in the United States. Without isoniazid (INH) prophylaxis, 2-3% of them ultimately will develop active disease.32
Drug resistance is substantially higher in the immigrant population. In many foreign countries, rates of drug-resistant TB approach 50%.33 The national TB surveillance system indicates that resistance to one first-line drug in the United States is approximately 18% in the foreign-born, vs. 9% in the United States-born.34 In patients who have received prior anti-TB therapy, rates of resistance skyrocket, reaching 31% in the foreign-born.34 Similarly, multi-drug resistant TB largely is concentrated in the foreign-born population; in 2000, 72% of multidrug-resistant TB occurred in immigrants.31
Clinical Spectrum. TB is caused by organisms of the Mycobacterium tuberculosis complex, which includes M. tuberculosis, M. bovis, and the attenuated form, Bacille Calmette-Guerin (BCG). M. tuberculosis causes the majority of disease; M. bovis is acquired through unpasteurized milk and found almost exclusively in the developing world.35 BCG is used as a vaccine and does not cause active disease.
The vast majority of TB cases in the immigrant population result from reactivation of latent disease.36,37 Strain typing demonstrates that TB in the foreign-born is almost universally an imported disease.36,37 After initial exposure, mycobacteria spread locally in the lung but usually are contained by the host’s immune system. Tubercles form and most of the bacilli die, after which the tubercles become calcified. Some tubercles, especially in the highly oxygenated areas of the upper lung, continue to contain viable mycobacteria for up to 20 years.38 The patient remains asymptomatic and noninfectious during the latent phase, and the only evidence of TB exposure is a positive skin test.38 A chest x-ray usually will be normal or may show a few calcifications in the hilar area or upper lobes, known as the "Ghon complex." Most immigrants with exposure to TB enter the United States during the latent period.
In subsequent years, as the host’s immunocompetence begins to wane, the immune system may lose its ability to contain bacilli. At this point, the disease may become active and bacilli may spread, affecting lung parenchyma or other organs via hematogenous dissemination. Reactivation TB most frequently happens during the first five years following migration.15-18 Contributing factors probably include increasing age and the onset of chronic debilitating diseases such as diabetes, neoplasia, or renal failure.38 The hallmark of active TB in immigrants is its insidious and nonspecific nature. Initial symptoms are constitutional and vague: night sweats, malaise, and anorexia. One study noted weight loss in 74% of patients and fatigue in 68%.35 When combined with language and cultural barriers, these early manifestations of active TB easily can be overlooked. People with advancing infection may develop a cough, which at first is nonproductive, but later is associated with blood-streaked sputum. Frank hemoptysis is unusual, and dyspnea typically occurs only with extensive lung destruction.35 Fever variably has been reported, occurring in 35-80% of patients.35
Although not extensively studied, there is some evidence that the immigrant population may have higher rates of extrapulmonary TB. In one study of Somali immigrants in Minnesota, 46% were found to have extrapulmonary involvement; the most common organ systems were lymph nodes, central nervous system (CNS), and bone.16 TB adenitis typically presents as painless cervical adenopathy, with or without cutaneous fistulas. In this study, 58% of extrapulmonary cases were acid-fast bacillus (AFB) smear negative but culture positive, and an additional 8% were both smear and culture negative.16 High rates of extrapulmonary TB also have been reported in Asian and Indian immigrants.39,40 Hence, health care providers must maintain a heightened suspicion of TB when evaluating immigrants from these regions of the world.
Diagnosis and Treatment. Diagnosis of TB usually includes some combination of purified protein derivative (PPD), chest x-ray, and sputum stain or culture. An important data point regarding TB in foreign-born individuals pertains to the patient’s BCG vaccination status. BCG is an avirulent attenuated strain of M. bovis that is used as a vaccine against M. tuberculosis. Clinical trials have shown substantial variability in the vaccine’s ability to prevent pulmonary TB (0-70%).35,38,41 In contrast, the vaccine appears very effective in preventing the serious sequelae of TB in young children, particularly disseminated TB and TB meningitis.35,38 For this reason, it still is widely used in developing countries and is recommended by the WHO. In some countries, 80% of children younger than 2 years of age receive BCG vaccine.35,38
BCG vaccination confers a variable degree of PPD positivity that wanes with time. Since it is given during childhood, many patients do not remember if they received BCG. A study of immigrants from various parts of Africa (i.e., Ethiopia, Liberia, Nigeria, Kenya, Zaire, and Somalia) found that 52% had positive PPDs and almost none of them knew their BCG status.41 More importantly, however, in endemic regions, data reveals no difference in the prevalence of positive reactions between those who reported having BCG vaccinations and those who said they had not.42 Thus, the CDC recommends that any history of BCG vaccination be disregarded when interpreting PPD results.43 The EP should use standard criteria for reading PPDs in immigrants. Induration greater than 10 mm is considered positive in those who have arrived within five years from a country with high rates of tuberculosis. A tuberculin skin test result greater than 5 mm is considered positive in patients with HIV, chest x-rays suspicious for the disease, or close contact to active TB.44
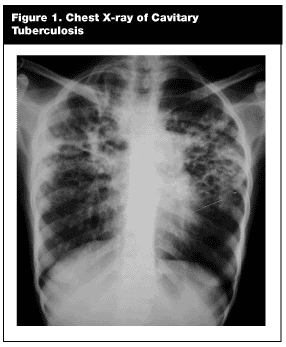
A chest x-ray should be performed in immigrants who report a positive PPD or symptoms of the disease. Reactivated TB has a predilection for the upper lobes of the lung, where it classically produces patchy or nodular infiltrates.45 Cavitation occurs in approximately 50%.45 In more advanced disease, fibrotic scarring develops, with a loss of pulmonary volume. (See Figure 1.) Immunosuppressed patients (i.e., extremes of age, HIV-positive, or with debilitating medical conditions) may have atypical findings; radiographs may show infiltrates in the lower lobes, a dissemination pattern, or even may be normal.45
Definitive diagnosis rests on AFB stain and culture of the sputum. Sputum culture is technically difficult and, thus, immigrants may receive only an AFB of their sputum upon entry into the United States. Sputum AFB is only 70% accurate45 and misses patients who are non-communicable (smear negative) but have active disease (culture positive). In one study, 53% of Tibetan patients later were identified with active TB after being missed at the first screening evaluation.17 Consequently, close follow-up of immigrants from high-risk countries is important. A single screening evaluation may not be sufficient. Diagnosis depends on a high degree of suspicion. Signs and symptoms of TB in the foreign-born may be frustratingly vague. Immigrants with reactivation disease usually do not have risk factors such as homelessness, alcohol abuse, or incarceration history. Further, most do not have any history of contact with active TB.36 Language barriers, lack of medical records, and the patient’s fears further complicate diagnosis. In short, the disease should be considered in the differential diagnosis of any immigrant with acute or chronic cough, weight loss, or vague constitutional symptoms.
Patients diagnosed with active disease should be hospitalized and kept in respiratory isolation until it is clear that they are non-communicable. The patient should receive multi-drug therapy until sensitivities are available to guide further treatment. Multiple studies have demonstrated rapid resistance to monotherapy using INH, up to 70% resistance rate within three months.38 Traditionally, the immigrant population has difficulty with compliance. Early hospitalization plays a crucial role in stopping further spread of disease.
Treatment of patients with latent infection (i.e., positive PPD) hinges on an analysis of risks and benefits. INH, the therapy of choice for latent disease, carries the risk of hepatotoxicity, anemia, gastrointestinal tract symptoms, and peripheral neuropathy.46 Risk of hepatotoxicity with INH increases from 0.3% in patients 20-24 years of age to 1.2% in 35-39 year-olds to 2.3-3% in those older than 50 years.45 Concurrent use of hepatotoxic drugs (e.g., acetaminophen), alcohol use, and chronic liver disease further amplify the risk. Nevertheless, treatment of high-risk individuals, including immigrants from TB endemic countries, is indicated regardless of age. The CDC recommends a nine-month regimen of INH with mandatory monthly assessments.47
Malaria. Epidemiology. The term malaria (literally "bad air") refers to the early belief that bad air of wetlands caused malaria. Now it is known that the "bad air" is actually the anopheline mosquito vector that causes infection with Plasmodium species.48 The earliest references to malaria were descriptions of febrile patients with splenomegaly from China in the Nei Ching Cana of Medicine in 1700 BC and from Egypt in the Ebers Papyrus in 1570 BC. During the 18th and 19th centuries, malaria was endemic in the United States and played a major role in the American Revolution and Civil War. More than 500,000 cases of malaria per year were reported in the United States in the first half of the 20th century, and the CDC originally was created as the Office of Malaria Control. Malaria remains a major cause of morbidity and mortality in tropical developing countries, particularly in sub-Saharan Africa. The WHO estimates 300-500 million cases and 1.5-2.7 million deaths occur annually.49 One million African children are killed by the disease yearly, dying mostly from cerebral malaria and anemia.49 Malaria is endemic in nearly every area of the world, including Asia, Africa, Central and South America, and the Middle East.49 In recent years, global incidence has been on the rise, due to combined effects of ecological change, drug resistance, and refugee migration.13
Malaria in the United States largely has been eradicated. Most malaria in the United States now consists of imported cases among immigrants, travelers, and military personnel. About 1000 cases of malaria are reported in the United States annually.50 In 1998, the CDC recorded a total of 1227 cases; 98% of these were imported, and one-third of all civilian cases occurred in foreign-born individuals.12 Within the immigrant population, more than 50% of cases were diagnosed in Africans, 24% in Asians, and 18% in people from Central America and the Caribbean.12
Malarial disease in humans is caused by four species of the protozoa plasmodium parasite: P. falciparum, P. vivax, P. ovale, and P. malariae.12,50-52 Each species has a distinct geographic distribution and, thus, the malarial species may be predicted based on recent travel history. P. falciparum predominates in Africa;12,50-52 it is associated with the most severe and complicated morbidity and causes the majority of mortality. P. vivax is the most prevalent species worldwide and predominates on the Indian subcontinent.12,50 Disease caused by P. vivax usually is less severe than P. falciparum and is characterized by a chronic, relapsing course. P. falciparum and P. vivax are evenly distributed in Latin America and Southeast Asia.12,50-52 P. malariae and P. ovale are common only in certain parts of Africa and cause much milder, even subclinical, malaria.12,50-52 Mixed infection is rare.13,52
A recent review of confirmed malaria cases in the United States found that the majority were caused by P. falciparum or P. vivax, with each species accounting for approximately 40% of disease.12 This may reflect detection bias, as the milder cases of P. malariae and P. ovale could go undetected by physicians who are unfamiliar with the disease. As one might expect, the majority of falciparum cases in the United States were imported from the African continent, whereas cases of vivax malaria were contracted in Asia, Latin America, and Africa.12
The importance of prompt diagnosis of malaria cannot be overemphasized. The disease begins with mild, nonspecific symptoms. If untreated, it is capable of progressing rapidly to life-threatening illness. Delay in diagnosis has been associated directly with increased mortality. Even in a modern intensive care unit (ICU) setting, the mortality of severe malaria is greater than 20%.53,54 Multiple studies have demonstrated that physicians in non-endemic countries, such as the United States, often fail to diagnose malaria on initial presentation.13,14,55 A retrospective review of falciparum malaria cases presenting to an ED in Los Angeles found that only 60% of cases correctly were diagnosed on initial presentation. Further, 16% of patients presented to a minimum of three doctors before the diagnosis of malaria was entertained.14 Similarly, in a Toronto study, an initial diagnosis of malaria was missed in 61% of patients presenting with falciparum malaria and 51% of patients with vivax malaria.13 In the same study, individuals with P. falciparum experienced an average delay of 7.6 days before correct treatment was initiated.13
Diagnosis of malaria may be difficult secondary to the nonspecific clinical nature of the disease. Fever, chills, headache, and malaise are common initial complaints. The periodic fever characteristic of malaria usually does not appear until the disease has been present for 1-2 weeks.56,57 Gastrointestinal symptoms of vomiting, diarrhea, jaundice, anorexia, and abdominal pain are common and may distract clinicians from the correct diagnosis. In one study, hepatitis and gastroenteritis were the most frequent misdiagnoses.14 Diagnosis in the immigrant further is complicated by the fact that many of these individuals are semi-immune.51,52 Immigrants who contract malaria fewer than two years after emigration usually have partial immunity to the disease, showing longer incubation periods and more subtle, nonspecific symptoms.52 In one study, 49 of 790 immigrants diagnosed with malaria were entirely asymptomatic; diagnosis of malaria was made incidentally on routine blood work.52 At the opposite extreme, expatriates who visit their native countries after years of living in the United States are non-immune. These individuals rarely use chemoprophylaxis (25-30%),14,52 and may develop severe disease with lethal complications. Altogether, 3.7% of the immigrant population develops severe complications with malaria.52
Life Cycle. Malaria is transmitted to a human host through the bite of an infected female anopheles mosquito.50,51 The sporozoite enters the bloodstream and migrates to the liver.50,51 Within hepatocytes, the sporozoites mature into merozoites or become dormant hypnozoites. After 1-2 weeks, the merozoites rupture the hepatocyte and enter the general circulation. Red blood cells (RBCs) are invaded.50,51 Inside the RBC, the merozoite uses hemoglobin as an energy source to continue maturation, a process that culminates in rupture of the RBC several days later.50 New merozoites are released to invade uninfected RBCs, and the cycle repeats.50,51 This intraerythrocyte cycle lasts 36 hours, except in P. malariae infections where maturation occurs over 76 hours.50 As a result, the patient experiences a periodicity of symptoms corresponding to intermittent parasitemia, with typical paroxysms occurring in 36-hour cycles.50,51
P. vivax and P. ovale have a unique ability to hibernate in infected hepatocytes as hypnozoites.50,51 In this dormant form, they may be asymptomatic for 6-11 months following the initial infection. Upon activation, hypnozoites mature into tissue schizonts and release infectious merozoites, producing a second symptomatic bloodstream infection. Common relapse causes include: pregnancy, surgery, travel, and other physiologic stresses.50
Clinical Spectrum. The clinical symptoms of malaria are due to the combination of parasitemia and subsequent hemolytic anemia. The timing of symptom onset varies with the species of plasmodium. P. falciparum has an incubation period of 8-30 days; therefore, infected individuals usually will become symptomatic in the first two months after arrival in the United States.13,14,51 One study noted a mean of 10 days from time of immigration to symptom onset.32 By contrast, the incubation period of P. vivax can be weeks to years because of the parasite’s ability to remain dormant in the liver.50,51 Nonetheless, most patients become symptomatic during the first two years in the United States; CDC statistics in 1998 showed that 98% of confirmed cases presented within a year after arrival.12
Infection begins with a flu-like prodrome: fever, malaise, myalgias, headache, nausea, and vomiting.50 At this stage, malaria resembles a viral illness. Not surprisingly, one of the most common initial misdiagnoses given to patients with malaria is viral syndrome. Within a few days, the patient begins to develop the paroxysms of malaria parasitemia. High fevers (up to 106°F in P. falciparum) lasting 3-4 hours are typical.35,51 The fevers are accompanied by headache, rigors, tachypnea, orthostatic hypotension, and prostration. Abdominal pain, vomiting, and diarrhea occur in 50% of individuals and may resemble gastroenteritis.14,51,52 The entire paroxysm lasts 9-10 hours, and between paroxysms the patient feels relatively well.51 Paroxysms occur frequently at first, but then establish a periodicity ranging between 48 and 72 hours.50 Non-immune patients are more likely to develop an overwhelming parasitemia with more severe symptoms.51,55
Physical examination of the immigrant with malaria may reveal mild jaundice with hepatosplenomegaly.51 Children, in particular, frequently have palpable spleens.51 Laboratory studies reveal anemia (due to hemolysis); however, severe anemia (Hb < 8 mg/L) occurs in only 3%.13,14 Leukopenia, thrombocytopenia (due to splenic sequestration), and mildly elevated transaminase levels are common.13,14 Sixty-four percent of patients will have a rise in lactate dehydrogenase levels.32 Hypoglycemia is ominous and tends to occur in children, pregnant women, and severely ill individuals.51
Complications. Of the four species infecting humans, P. falciparum is responsible for nearly all of the severe complications and mortality resulting from malaria. Unlike other plasmodium species, the merozoites of P. falciparum infect erythrocytes of all ages, causing severe parasitemia. The semi-quantitative relationship between the magnitude of parasitemia and death likely is related to more severe microvascular damage and metabolic derangements (hypoglycemia and lactic acidosis).58,59
Cerebral malaria is the most dreaded complication of acute malaria. Usually seen in children ages 3-6 years, it may occur in non-immune adults with severe infection.14,55,60 Symptoms arise due to the sequestration of infected erythrocytes within cerebral capillaries and venules.60 After several days of malaria paroxysms, the affected individual abruptly develops mental status changes, lethargy, and then coma.14,55,60 Signs may include decorticate posturing or opisthotonos. Generalized seizures occur in 20-50% of patients.51,60 A head computed tomography (CT) scan will demonstrate diffuse edema.60 Mortality from cerebral malaria ranges from 20% to 50%.55,60 There is no specific treatment aside from anti-malarial chemotherapy and supportive care.
Acute renal failure is a commonly seen complication in adults.55 It is an acute tubular necrosis due to hemoglobinuria.51 Blackwater fever (BWF) is a syndrome of severe intravascular hemolysis and hemoglobinuria resulting in acute renal failure. Classically, BWF occurred in European expatriates chronically exposed to P. falciparum and irregularly taking quinine. The disease disappeared with the introduction of chloroquine but has experienced a recent resurgence with the increasing popularity of anti-malarials other than chloroquine.54,56
The patient with renal failure may require hemodialysis, but with resolution of the parasitic infection, this condition usually is reversible. Finally, acute hemolysis may cause an anemia severe enough to be life-threatening. This complication usually is seen in children younger than 3 years of age.
Diagnosis. Examination of blood smears for parasitized erythrocytes remains the gold standard for diagnosis of malaria.50,51 First, a thick smear is made, optimizing the chance of detecting parasitemia.50 The technique is simple but must be done by hand; automated machines typically miss the parasites.51 If plasmodia are identified on thick smear, a thin smear is prepared to identify the strain and quantify the level of parasitemia.50 Symptoms of infection may precede the appearance of parasitized RBCs by several days. Therefore, repeat smears (every eight hours) are required to exclude the diagnosis of malaria completely.50,51
Several serological tests are available for diagnosis of malaria infection. The newest antigen-based dipstick assay, OptiMAL, is capable of differentiating between P. falciparum and P. vivax, as well as current or past infection.61 One study suggested a 100% specificity and 95% sensitivity for both species.62 Older dipstick assays were problematic because they detected only P. falciparum.61 All assays remain expensive and further studies are needed to clarify their sensitivity.61 They may be helpful when experienced microscopy is not available in a timely fashion.50
Treatment. Malaria infection, especially P. falciparum, is a medical emergency. Successful treatment requires early and aggressive chemotherapy. All patients with P. falciparum should be hospitalized for treatment.50,51 Individuals infected with P. vivax need hospitalization if pregnant, immunocompromised, or significantly ill;51 however, well-appearing individuals may be discharged with close follow-up. Occasionally, a patient with a negative blood smear despite a concerning clinical story may need admission for serial smears.
Mounting antimalarial resistance is the No. 1 factor in the worldwide resurgence of malaria.63 Therefore, the clinician must know current treatment trends used in different regions of the world. If P. falciparum infection can be excluded, patients may be treated with an outpatient regimen of oral chloroquine phosphate. (See Table 4 for dosages.) Adequate therapy is noted with defervescence and a decreased level of parasitemia within 72 hours from induction of therapy.50 Many hospitals in the United States do not carry chloroquine; in this case, hydroxychloroquine may be substituted. With completion of initial therapy, patients infected with P. vivax and P. ovale should receive primaquine phosphate to eradicate latent forms in the liver.64 Of note, primaquine is a powerful antioxidant and is contraindicated in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.50 Chloroquine also may be used for patients who acquired P. falciparum in Central America, Haiti, or the Middle East. Patients with P. falciparum from all other areas of the world should be considered chloroquine-resistant. In these patients, quinine is the drug of choice.49,50 Quinine should be given orally for a total of seven days.50,51,55 (See Table 4 for dosages.) In some areas of Southeast Asia, plasmodium with decreased sensitivity to quinine exists. Immigrants from these countries will require a 10-day course of quinine.49,50 Traditionally, an antifolate drug, such as sulfonamide/pyrimethamine or a tetracycline is co-administered with quinine to prevent a small rate of treatment failure.50,51,64
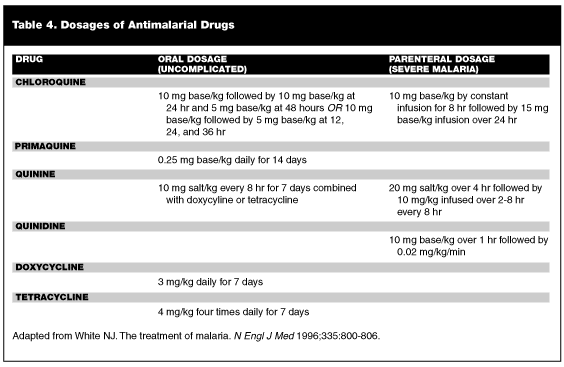
Patients who are unable to tolerate oral medication, in whom greater than 5% of erythrocytes are parasitized, or who have associated organ dysfunction (i.e., renal failure, adult respiratory distress syndrome [ARDS], or cerebral malaria) should receive parenteral therapy.50 Quinine no longer is licensed in a parenteral form in the United States. The CDC recommends quinidine for treatment of malaria in these patients.50,51,55 (See Table 4 for dosages.) The infusion is continued until the patient can tolerate oral quinine. Patients should be monitored for any signs of QT prolongation or QRS widening.50,51 Complications of malaria are managed expectantly. Hypoglycemia is common with cerebral malaria and in pregnant patients, and may be precipitated during quinine therapy.50 Severe anemia may require transfusions, and patients with ARDS should receive mechanical ventilation as indicated. Corticosteroids no longer are advised in the treatment of cerebral malaria.64 Gram-negative bacteremia may complicate P. falciparum malaria; therefore, the patient vigilantly should be monitored for signs of gram-negative bacteremia. Finally, exchange transfusions may be done for especially severe parasitemia.50,51
Intestinal Parasites
Epidemiology. Intestinal parasitosis is common among immigrants. Parasitic infections are a worldwide problem, but the heaviest burden of disease is carried by the developing world. Of the 1 million naturalized United States citizens in 1996, an estimated 600,000 came from countries where intestinal parasites are endemic.65 In 1998, the CDC’s Division of Quarantine found that 30% of all immigrants who underwent screening exams showed evidence of intestinal parasite infection.3 The highest rates were seen among Southeast Asians (40%) and Africans (45-60%).2 Infection by multiple pathogens is not uncommon.66,67 The most frequent pathogens vary slightly with country of origin. In Central American refugees, Trichuris trichiura, Giardia lamblia, and Ascaris lumbricoides made up the majority of infections, while hookworm was relatively rare.66 In Cambodian and Hmong patients, Giardia, Hymenolepis nana, hookworm, and Strongyloides stercoralis appeared to be the most prevalent parasites.67 As a general rule, worm burden is greatest in children, for whom symptomatology and public health risk also are most pronounced.67
Clinical Spectrum. Human intestinal parasites are transmitted via ingestion of food or water contaminated by human or animal feces. All intestinal parasites are capable of causing diverse gastrointestinal (GI) symptoms. Common complaints include abdominal cramps, bloating, anorexia, diarrhea, weight loss, nausea, and vomiting.3,67 Some patients are completely asymptomatic. Of note, the presence of symptoms has not been shown to correlate well with degree of intestinal parasitosis.66,67 Multiple studies have shown that symptoms in a parasitized host may represent heavy infection or be coincidental.68-70 In immigrants who have had negative work-ups for chronic abdominal pain, it may be helpful to check stool studies or to treat presumptively for parasite infection. The true relevance of parasite infection to the EP lies in the complications of parasitosis. These complications cause significant morbidity and may be life-threatening.71 The classic complications associated with each individual species of parasite are listed in Table 5.
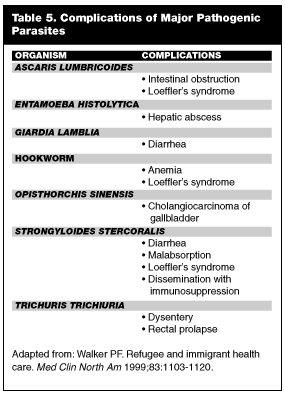
A common complication of intestinal parasite infection is an eosinophilic pneumonitis known as Loeffler’s syndrome.71,72 Pulmonary migration of larvae in some patients provokes an intense allergic response consisting of fever, cough, wheezing, and dyspnea.3,72 The clinical picture resembles an asthma exacerbation or an interstitial pneumonia. A chest x-ray of a patient with Loeffler’s syndrome may show an infiltrate.71 Laboratory studies usually reveal an increased peripheral eosinophil count (12-14%).71 Treatment rests on identification of the culprit parasite; dosages are the same as in intestinal infection.
Ascaris (Roundworm). Ascaris currently is the most prevalent helminth worldwide. Approximately one-quarter of the world’s population is infected with Ascaris. In Africa, Central America, South America, and the Far East, the incidence may be as high as 90%.71 Infection is due to fecal-oral passage of eggs from contaminated hands.72 After hatching in the small intestine, larvae migrate to the pulmonary circulation and then are coughed up and swallowed, where they attach to the small intestine for the remainder of their life cycle.72 In the immigrant population, Ascaris usually is an imported infection, and clinical manifestations typically occur fewer than three years following arrival.66,67,72 This time frame reflects the lifespan of the adult worm in the small intestine. After three years, most infections will resolve spontaneously.72
The EP occasionally will encounter the patient who complains of passing worms in his stool or, worse, through the nose or mouth. Figure 2 depicts worms found in the diaper of a child from El Salvador. Such an occurrence always is a result of Ascaris infection; hookworm and whipworm do not cause formed worms to be passed in the stool, and pinworm usually is too small to be noticed.72 If roundworm infection proceeds uninterrupted, a tangled mass of luminal adult Ascaris may form, causing serious mechanical bowel problems. Small bowel obstruction, intussusception, volvulus, or perforation may ensue.72 Children, in particular, are susceptible to bowel complications secondary to smaller gut diameter and higher worm burden.
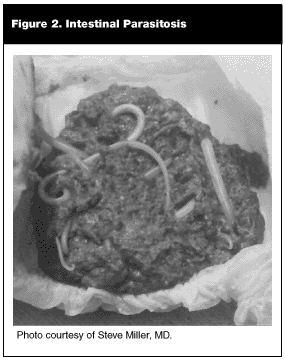
Stool examination for ova and parasites is the best method for confirming the diagnosis of ascariasis.71 Uncomplicated cases may be treated by a single dose of albendazole (400 mg orally) or a three-day course of mebendazole (100 mg twice daily for 3 days).72 Surgery may be necessary to relieve intestinal obstruction. Occasionally, Ascaris worms will migrate into the biliary tree, causing cholecystitis, cholangitis, or pancreatitis.71,72 In addition to anti-parasitic therapy, endoscopic retrograde cannulation of the pancreatic duct (ERCP) usually is required to alleviate the obstruction.71,72
Enterobius. Enterobius (pinworm) infection classically is associated with perianal itching.72 Adult worms live in the small intestine and colon and migrate nocturnally to the perianal skin to lay their eggs, causing an intense pruritis.72 Auto-reinfection is common as the patient scratches and ova lodge under the fingernails.72 Patients infected with pinworm will not demonstrate an eosinophilia because the worm’s life cycle is confined to the GI tract; diagnosis easily is confirmed by the transparent tape test.72 A strip of tape is applied to the perianal skin in the morning and then examined microscopically for the presence of Enterobius ova. Treatment with albendazole (400 mg orally) or mebendazole (100 mg twice daily for 3 days) usually is very effective but requires two courses of therapy to prevent auto-reinfection.72
Trichuris trichiura. Trichuris (whipworm) is a parasite that attaches to superficial cecal and colonic mucosa of infected patients. Like Enterobius, the life cycle of the whipworm is confined to the GI tract; therefore, it rarely results in eosinophilia.72 Trichuris infection may cause abdominal pain, anorexia, bloody diarrhea, and can even mimic inflammatory bowel disease.72 The main clinical significance of whipworm infection, however, lies in the propensity of heavy infections to cause rectal prolapse, particularly in children.3,72 Examination will reveal multiple threadlike worms embedded in prolapsed rectal mucosa.72 Treatment consists of either albendazole (400 mg orally) or mebendazole (100 mg twice daily for 3 days).72
Hookworm. Hookworm infection occurs in immigrants from warmer climates, especially Southeast Asia. Worldwide, hookworm infection remains the most common cause of iron deficiency anemia.3,73,74 Necator americanus predominates in the Americas, the Caribbean, southern Asia, and sub-Saharan Africa, while Ancylostoma duodenale is prevalent in North Africa and parts of Eurasia. In some tropical countries, both types of hookworm may be found. Infection begins when the larvae burrow through the skin of the feet or hands. The infective larvae then travel sequentially through the lungs and into the GI tract, where they attach to the wall of the small intestine and consume blood. The parasite causes a significant anemia and can reside for up to six years in the small intestine.67 Patients may present with pica, fatigue, angular stomatitis, and hemoglobin levels of 3-8 g/dL.
Confirming the presence of hookworm ova in stool is diagnostic, but multiple examinations may be required since egg production is scant. A helpful clue for the EP is the presence of peripheral eosinophilia, which is extremely common in hookworm infection. In one study, hookworm ultimately was diagnosed in more than half of immigrant Southeast Asians presenting with persistent eosinophilia, despite three negative stool examinations.72-74 Treatment consists of either albendazole (400 mg orally) or mebendazole (100 mg twice daily for 3 days).
Strongyloides. Finally, Strongyloides infection in the immigrant deserves special mention. Like hookworm, Strongyloides infection begins when the worm burrows through the skin of the hands or feet and migrates through the lungs to the small intestine, where it attaches to the mucosal wall.3 This worm is unique in its longevity, residing up to 40 years in the GI tract. In one classic study, British prisoners of war were found to have ongoing Strongyloides infection several decades after their release.75
The parasite may cause a hyperinfection syndrome in the presence of immunosuppression.76 Most individuals infected with Strongyloides remain asymptomatic for years, manifesting a fluctuating eosinophilia.72 Some individuals may experience a mild Loeffler’s syndrome, and nonspecific abdominal pain is common. Larva currens ("racing larvae") is a pathognomonic skin eruption caused by Strongyloides larvae. The migrating larvae create urticarial serpiginous tracks, usually of the buttocks, groin, and trunk. The rash typically lasts only hours, but may recur over a period of weeks to years.72
In the setting of immunosuppression, Strongyloides has the unique ability to penetrate the bowel wall and disseminate widely into virtually every organ in the body.77 The result is a severe sepsis syndrome with mortality of 60-80%.3,72,76 Disruption of the bowel mucosa by the worms induces abdominal pain, vomiting, and diarrhea. Sepsis from gram-negative bacteria often ensues, probably due to passive carriage of bowel bacteria with worm invasion.72 As dissemination progresses, the patient develops multi-organ dysfunction with ARDS, renal failure, meningitis, and hepatitis.72,76 Interestingly, eosinophilia is uncommon during the hyperinfection syndrome. Death usually is due to bacterial superinfection.72,76
Thus, some physicians recommend that patients with eosinophilia from highly endemic regions (especially Southeast Asia) be treated empirically for Strongyloides infection if they are to receive high-dose steroids or other immunosuppressive regimens.3 Obtaining a definitive diagnosis of Strongyloides infection is difficult. Stool studies usually are unhelpful since the worm does not produce many ova. Only 30% of infections will be detected by a single stool sample.72 The most consistently helpful clue is the presence of eosinophilia unless the patient has developed hyperinfection. Ultimately, diagnosis may be confirmed by serum antibody assay.35 Treatment of Strongyloides differs from that of other intestinal parasites. It is accomplished via thiabendazole 25 mg/kg twice orally for three days or multiple-dose albendazole (400 mg daily for 3 days).35,38
Hepatitis B
Epidemiology. Hepatitis B is a major cause of chronic hepatitis, cirrhosis, and, in many areas of the world, hepatocellular carcinoma.78 Modes of hepatitis B transmission include perinatal, sexual (semen and vaginal secretions), and via contaminated blood products. The disease affects approximately 350 million people worldwide. Serological studies indicate that Southeast Asia, sub-Saharan Africa, and the Western Pacific have the world’s highest infection rates. In these areas, 50-80% of the general population shows evidence of prior hepatitis B infection.79,80 In the United States, the prevalence of hepatitis B among foreign-born individuals reflects infection rates in the country of origin.3 Evidence of prior hepatitis B infection exists in approximately 80% of Southeast Asian immigrants, while chronic infection exists in 14%.81,82 Similarly, more than 70% of refugees from East Africa have positive hepatitis B serologies, and 11-12% are chronic carriers.83,84 With the recent wave of refugees from Afghanistan, limited data about the prevalence of hepatitis B in this ethnic group now are available. An estimated 14% of Afghan immigrants are chronic hepatitis B carriers.84,85 The virus is not endemic in immigrants from the former Soviet Union, who show carrier rates similar to that of the non-immigrant United States population (0.4-1.5%).84,86
Rates of hepatitis B remain elevated in the United States-born children of immigrants due to both vertical and horizontal transmission. The significance of vertical, or perinatal, transmission is well recognized; population studies demonstrate infection in 55% and chronic active disease in 30% of children born of infected mothers.87 There is mounting evidence that horizontal transmission also may be an important route by which hepatitis B is acquired.87-89 United States-born children of uninfected immigrant mothers have hepatitis B rates that are 4-8 times greater than that of the general population.87-89 These cases are thought to occur horizontally by inoculation of skin and mucous membranes and typically transpire in the first two decades of life. A common Hmong remedy for illness, for example, involves the pricking of fingertips with a sewing needle.87 As refugees frequently live in large, crowded, extended family units, even children with no infected immediate household members are at higher risk for acquiring the disease.87,88
In contrast to the United States, where infection typically occurs during adulthood, most of the hepatitis B in developing countries is acquired in the first two decades of life.87-90 This has important clinical implications. In hepatitis B infection, the risk of chronic disease correlates inversely with the age at exposure.35,91 Adults who are exposed to hepatitis B have carrier rates of less than 5%; however, persistent disease occurs in more than 90% of infected neonates.35,90,91 Approximately 25-50% of children who are infected in the first five years of life become carriers.91 This is believed to result from the inability of the child’s body to mount an immune response; the inflammatory reaction is responsible for both the symptoms of acute infection as well as the ultimate production of antibody.35 Thus, neonates and children usually are asymptomatic during acute infection, contrasting with the adult population, who manifest symptoms such as jaundice, vomiting, right upper quadrant pain, and weight loss.35,91 Adults are far more likely to produce antibody to the disease and have lower carrier rates.
Clinical Spectrum. The EP rarely will encounter acute hepatitis in the immigrant. Most foreign-born individuals with hepatitis B acquired the disease during childhood and do not recall their acute infection. Instead, the immigrant typically presents with the numerous long-term complications of chronic hepatitis.
Individuals who acquire hepatitis B in the first two decades of life are at greatest risk for the development of cirrhosis and hepatocellular carcinoma at a young age.92,93 Hepatomas have been reported in children as young as 6 years of age.3 The risk of hepatocellular cancer is 400 times higher in patients with chronic hepatitis B. In highly endemic areas, hepatomas remain the leading cause of cancer-related death.91 One study of Chinese and Japanese immigrants in the United States noted that the overall rate of hepatocellular cancer was 26/100,000 per year, compared with a rate of 3/100,000 per year in the United States-born, non-ethnic population.93 Another study noted that the rate of progression to hepatocellular carcinoma in chronic hepatitis B infection is 2% at five years, 5% at 10 years, and 18% at 15 years.90 Ultimately, one-third of the people who acquire hepatitis B as children will die of either cirrhosis or hepatocellular cancer.3 Therefore, the clinician is highly likely to encounter immigrants with the protean manifestations of chronic liver disease. These may range from a benign hepatitis flare with elevated liver function tests and right upper quadrant pain to life-threatening complications such as GI bleeding, encephalopathy, or spontaneous bacterial peritonitis.
Many immigrants will not know their hepatitis B status. Good screening tests for hepatic dysfunction in the ED are a urine dip for bilirubin or serum aminotransferase levels.91 If either is elevated, further work-up is warranted. Bilirubin and alkaline phosphatase levels will be elevated mildly in chronic hepatitis, but significant increases suggest a complication within the biliary tree.91
A prothrombin (PT) level is a good screen for synthetic liver dysfunction and identifies patients at higher risk of bleeding complications. The presence of altered mental status may indicate encephalopathy. Abnormal renal function tests may reveal hepatorenal syndrome.91 Be cognizant of the high risk of hepatoma in these patients; any history of weight loss, gradually increasing right upper quadrant pain, or jaundice warrants abdominal imaging (typically an ultrasound) for the detection of masses. Ultimately, the diagnosis of hepatitis B is made via a standard serology panel, which includes surface antigen (HbsAg), surface antibody (HbsAb), and core antibody (HbcAb).
Treatment is dictated by patient presentation. Complications of liver failure should be managed according to standard protocols, such as parenteral antibiotics, endoscopy, or lactulose therapy. Patients with surface antibody are considered immune and have minimal risk for long-term complications. Those patients who are chronic carriers but have normal transaminases should be followed periodically by a PMD but do not generally require specific therapy. In contrast, patients who have evidence of chronic infection (HepSAg+) in association with elevated transaminases may be candidates for specific anti-hepatitis therapy. Treatment with interferon alfa is recommended for patients with persistent elevations in serum aminotransferase concentrations, detectable levels of HBsAg, HBeAg, and HBV DNA in serum, chronic hepatitis on liver biopsy, and compensated liver disease.94 Another promising treatment is the use of one or multiple nucleoside analogues, such as lamivudine.95
Some programs screen HbsAg-positive individuals every six months with an alpha-fetoprotein level and right upper quadrant ultrasound for hepatocellular cancer. In the past, liver transplant has not been performed due to the fact that the virus usually infects the graft, resulting in poor outcome.90 Recent preliminary studies indicate that a combination of hepatitis B immunoglobulin and lamivudine may decrease rates of transplant rejection to acceptable levels.90 Further studies are needed on this issue.
The cornerstone of hepatitis B control is prevention through vaccination. The current 1982 vaccine is 85-90% effective in preventing transmission in children, healthy adults, and pregnant women.96 The United States now has a policy of universal infant hepatitis B vaccination.96 Epidemiological studies of pregnant Asian Americans show that 2% are chronic hepatitis B carriers, vs. 12% for pregnant foreign-born Asian women.97 The vaccine should be given with hepatitis B immunoglobulin (one-time dose) to the neonates of these women within 12 hours of birth and again at 1 month and 6 months.
Mental Illness: Post-Traumatic Stress Disorder and Somatization
PTSD and somatization are both well-documented among immigrant and refugee populations. Multiple studies have documented a higher incidence of mental disease processes in the foreign-born population as compared with United States-born non-ethnic individuals.97-100 The higher prevalence of PTSD and somatization has been attributed to events that occur before departure (i.e., war, torture, famine, and poverty), during the migration process itself (i.e., loss of family and home, further trauma such as robbery or rape), and after arrival in the United States (i.e., language barriers, and cultural adaptations).26,101 For the EP, psychiatric disease may be one of the most frustrating and troublesome disorders encountered. First, it usually is a diagnosis of exclusion, frequently requiring an extensive work-up to rule out organic disease.102 Furthermore, patients may be reluctant to accept a psychiatric diagnosis due to the stigma associated with it.103,104 PTSD and somatization more frequently occur in older individuals with co-existent medical illnesses, thus confounding the diagnosis.103,104 Language barriers and cultural idioms make the diagnosis of psychiatric disease even more difficult for the immigrant. An understanding of the immigrant’s cultural perspective is extremely helpful in overcoming some of these barriers.
Post-traumatic Stress Disorder. Modern research on PTSD began with studies of Jewish survivors of Nazi concentration camps, who suffered from "concentration camp syndrome." It was characterized by fatigue, irritability, anxiety, and depression.3 In 1975, a wave of refugees from Indochina prompted renewed interest in the psychiatric effects of severe trauma and migration.105,106
By definition, PTSD occurs in response to severe stressors and, as a general rule, the more severe the stressor, the more likely a person is to develop PTSD.24,104 As expected, refugees who have fled situations of war and ethnic strife have a higher prevalence of PTSD than immigrants from more stable areas.24,104 One study of Cambodian refugees, including many Pol Pot concentration camp survivors, noted that 80% met DSM-IV criteria for PTSD a decade after their arrival in the United States.103 These immigrants had endured threats to life, forced labor, beatings, starvation, and had witnessed the deaths of family and friends.103 More recent immigrants from Eastern Europe have received attention; a 1998 study of displaced Bosnian women documented PTSD in 53%.107 Even foreign-born individuals who have not suffered significant pre-migration stress are at increased risk for PTSD. In a study of Central American and Mexican immigrants, the Mexican individuals who had no history of prior trauma or torture displayed rates of PTSD of 25%. A rate of 1-8% for PTSD exists in the general population.103
PTSD traditionally is considered a coping and processing mechanism in the face of extreme stress. The three cardinal elements of PTSD are re-experiencing, avoidance, and hyperarousal.7,24,38 Re-experiencing occurs in the form of flashbacks, nightmares, or extreme distress in response to events that are reminiscent of the original trauma.7,24,38 Avoidance symptoms include an inability to remember traumatic events, diminished affect, and a "sense of foreshortened future."7,24,38 Hyperarousal has been described as excess irritability, insomnia, and difficulty concentrating.7,24,38 The symptoms of PTSD tend to be complex and vague. Patients may not be forthcoming about their history and referral to a psychiatrist experienced in both trauma and the treatment of refugees may be required to make the diagnosis.
Somatization. Closely related to PTSD is somatization. Somatization may occur without organic pathology or may augment existing physiologic complaints. These patients habitually over-utilize medical systems and undergo time-consuming, unnecessary, and often invasive work-ups that are frustrating to all parties involved.102 Any individual in cultural or geographical transition is at increased risk for somatization, particularly if their ethnic group discourages direct expression of emotional distress.108
A review of Asian immigrants seeking care at a Seattle health clinic noted that 25% of visits were for "vague somatic symptoms," such as abdominal pain, chest pain, dizziness, flatulence, headache, shortness of breath, back pain, decreased appetite, and insomnia.102 These symptoms occurred without a clear-cut organic etiology and were more expensive to evaluate than visits for clearly physical or mental disorders.102 Patients in this study were divided into immigrants who had left their home country voluntarily and refugees who were forced to flee their homes. Somatization was greatest in the refugee population.102 Another study of 966 Soviet immigrants to Israel noted an elevated prevalence of somatization of 22%, when compared to nonimmigrants.109 Chest pain, weakness, and nausea were the common complaints.109 Somatization tends to increase with the age of the patient and with the length of time following resettlement. It frequently occurs in patients with concurrent medical conditions and, therefore, may be difficult to assess. In one study, only half of the psychiatric disorders that presented somatically were diagnosed correctly on initial presentation.26 Consequently, somatisizers tend to have more frequent and expensive medical visits.109
Reasons for somatization in the immigrant population are complex and best understood in the context of cultural expression. To begin with, non-Western cultures usually do not separate the physical and the emotional. This paradigm is unique to Western culture.26 The perceived link between body and mind reflects ethnicity and religion and can vary even among patients of similar geographic background. For instance, the Mien language has no words for mental illness, so symptoms of anxiety and depression must be expressed in somatic terms.7 At a psychiatric clinic in Oregon, 93% of Mien patients who had been diagnosed with PTSD complained of headache, while 81% complained of back pain and 71% complained of dizziness.101 Of 75 patients, not a single person ever voluntarily admitted to psychiatric symptoms.101,103 Complicating their diagnosis was the history of closed head injuries and back trauma sustained during years of interrogations, threats, and forced relocations.101,103
Southeast Asians suffering from depression often will complain of a "weak heart," "weak kidney," or "weak nervous system."26 Interestingly, it appears that foreign-born individuals develop a more Western attitude toward psychological illness with acculturation.26,101 One study of Hispanic women noted that those with low acculturation were most likely to complain of somatic symptoms when faced with stressful situations, as opposed to more acculturated women who reported depression before somatic symptoms.26 Compounding the problem, some cultures view psychological problems as inappropriate. Mental illness may not be viewed as a legitimate disease. It may be stigmatized, as in the Chinese and Vietnamese cultures.102,104 These individuals are much more likely to use somatic idioms such as "I have a headache" rather than complain directly of sadness or anxiety; somatic complaints engender more social support and provide an acceptable reason for seeking medical care.102,104 Immigrants may worry about deportation if they are thought to be "crazy."3,7
EPs directly should question the patient about symptoms of mental health disorders. Most somatisizers can admit to anxiety and depression when asked.101 If available, immigrants should be referred to culturally sensitive treatment centers.103,110 A combination of Western and traditional medicines often is required, including shamanic healing or religious ceremonies.101 At a psychiatric treatment center for Mien patients, doctors found that patients were hesitant to take a medicine for what they believed was a problem of the spirit world.101,103 These patients felt that Western medications were helpful for diseases of organic pathology, but a shaman was necessary to appease the spirits.101 Pharmacological therapy may involve the use of antidepressant medications. Clonidine and tricyclic antidepressants (TCAs) have been reported helpful in patients with PTSD by reducing the incidence of nightmares, irritability, and startle responses.103 Family members should be involved in the treatment plan, as the family is the center of identity and stability in many cultures.26,101 Since much of the psychological rift stems from cultural discordance, therapy is most successful when a cross-cultural approach is used.
Underimmunization
Epidemiology. When interviewing a foreign-born individual, it is important to document the patient’s immunization history. Most have not received a complete primary series by United States standards. Data from the CDC’s Division of Quarantine in 1998 indicated that 51.5% of new arrivals had an incomplete vaccination series.3 Most immigrants receive some diphtheria, pertussis, tetanus, and polio coverage in their home countries, but vaccination for measles, mumps, and rubella is much more sporadic.7,86,111 One survey of Guatemalan and Salvadoran refugees in Belize indicated that only half of the children had received measles immunization.111 Studies of immigrant children in the United States revealed that H. influenza B (Hib) and hepatitis B vaccinations were lacking even more frequently.112 In one survey of Latino children at a New York immunization clinic, Hib coverage was 12.5% for foreign-born children in comparison to 78.5% for United States-born children.112
Underimmunization in the foreign-born reflects decreased surveillance and access to care, and local vaccination patterns.111 For example, rubella vaccine was not given standardly in countries of the former Soviet Union, and administration of measles/mumps vaccine was sporadic at best.26,86 A survey of refugees from the areas of Ukraine, Russia, and Belorussia found that 14% lacked measurable antibody to measles, mumps, or rubella.86 Negative test results were most likely in those younger than 30 years old, suggesting that older immigrants had acquired immunity from having the disease.86 Many immigrants do not know if they have received a full vaccination series. Obtaining documentation in the foreign-born can be notoriously difficult. A recent study of internationally adopted children found that 65% had no written records of overseas immunizations.113
Measles. Measles (rubeola) is a highly contagious viral illness transmitted via respiratory secretions. Routine use of the measles vaccine has eliminated the disease in the United States except for sporadic outbreaks.114 After a 1990 epidemic with 27,000 cases, the CDC instituted a policy of two doses of measles vaccine for every child; the first dose is administered between 12 and 15 months and the second at age 4-5 years or age 12 years.114-116 This resulted in near universal coverage of United States-born children; measles now largely is limited to the foreign-born.114,116 During the year 2000, 62% of reported cases were associated with imported cases.116 The countries most frequently implicated were Japan, Korea, Ethiopia, China, and Vietnam.116 For many reasons, children of foreign-born parents also tend to be under-immunized and disproportionately affected during an epidemic. During the 1990 epidemic, Hispanic patients accounted for 45% of cases in Washington state.117
EPs should be familiar with the clinical presentation of measles; prompt recognition is crucial to prevent spread to other patients. In the 1990 epidemic, 17% of all cases reported in Washington occurred through exposure in medical settings and most of these were in the ED.117 Nine children presenting to various EDs initially were misdiagnosed as hepatitis, viral syndrome, drug reaction, or Kawasaki disease.117 These patients remained in the EDs for up to 13 hours and subsequently were either hospitalized without isolation or sent home. At least 31 secondary cases occurred in family members, other patients, visitors, or hospital staff from these exposures.117
Measles begins with a classic prodrome of dry cough, conjunctivitis, coryza, and increasing fever (up to 105° F).35 At this stage, symptomatology often resembles influenza.35 The appearance of Koplik spots, white spots located on the buccal mucosa just adjacent to the molars, is pathonogmonic for the disease and can aid in clinical diagnosis.38 Between days 2 and 4, an erythematous maculopapular rash develops, beginning at the hairline and behind the ears and then gradually spreading downward to encompass the entire body.35,38 After day 4, the rash begins to disappear in the same order in which it appeared and may desquamate.35 Fever begins to abate around the same time (days 6-7).35,38 Diarrhea and vomiting are common throughout the course of the disease.88 Other complications occur secondary to destruction of mucosal surfaces, including stomatitis, laryngitis, and bronchitis.118 Secondary bacterial infections also are common; prolonged fever may indicate otitis media, mastoiditis, or pneumonia.118 Labs typically reveal a lymphopenia, and a chest x-ray may show an infiltrate. Hospitalization and mortality from measles usually result from pneumonia with respiratory failure and other infections.118
Treatment of the disease remains largely supportive. Several trials have suggested that high doses of vitamin A may prevent mortality in severe measles, particularly in children younger than 2 years.35,38 The American Academy of Pediatrics (AAP) Red Book for 2000 recommends vitamin A supplementation in certain subgroups of patients, including children 6 months-2 years of age and children with recent immigration from areas with high mortality rates due to measles. Doses are 50,000 IU for infants 1-6 months; 100,000 IU for infants 7-12 months; and 200,000 IU for children older than 1 year.35,38
Measles generally is diagnosed on clinical grounds. Confirmation is made by serum IgM levels.38 If a suspected case is encountered, care must be taken to prevent secondary cases. The virus is phenomenally contagious, with epidemics originating from one or two index cases. Casual contact at school, in a doctor’s office, or on a bus is usually enough for disease transmission to occur. Secondary cases occur in children who have not yet received the vaccine or in adults who never were immunized, a phenomenon common in inner city populations and immigrant populations.116,119 Primary vaccine failure may account for some of these cases.115 Any susceptible children or adults who have been exposed to measles should receive immunoglobulin. It has been shown to exert a protective effect up to six days after exposure.35,38,118
Rubella. Despite widespread administration of the measles, mumps, rubella (MMR) vaccine in the United States, outbreaks of rubella continue to occur, largely among adults born in countries that do not routinely vaccinate against the disease. In 1999, 86% of rubella cases in the United States occurred in Hispanic women; most were from Mexico and Central America.120 Similarly, 81% of congenital rubella cases occurred in infants of Hispanic women.120
Vaccination of susceptible mothers can be challenging because they often have little or no contact with the United States health care system. Nonetheless, all non-pregnant, susceptible individuals born after 1956 are eligible for vaccination with the standard MMR vaccine.96 Many individuals will not know their vaccine history; however, there is no evidence to suggest adverse outcome from giving MMR to someone who is immune from natural disease or prior vaccination.96
References
1. Lollock L. The foreign born population in the United States: March 2000. Current Population Reports. Washington, DC: United States Census Bureau; 2001:20-534.
2. Lillie-Blanton M, Hudman, J. Untangling the web: Race/ethnicity, immigration, and the Nation’s health. Am J Public Health 2001; 91:1736-1738.
3. Walker P, Jaranson J. Refugee and immigrant health care. Med Clin North Am 1999;4:1103-1120.
4. Kramer E, Ivey S, Ying Y. Immigrant Women’s Health. San Francisco; Jossey-Bass Inc:1999.
5. Statistical abstract of the United States, 1996. In: The National Data Book. 116th ed. Washington, DC: U.S. Department of Commerce, Bureau of the Census;1996.
6. Protocol relating to the status of refugees. Message from the President of the United States transmitting the protocol relating to the status of refugees. New York, Jan. 31, 1967. Washington, DC: U.S. Government Printing Office; 1968.
7. Gavagan T, Brodyaga L. Medical care for immigrants and refugees. Am Fam Physician 1998;57:1061-1068.
8. Grieco E, Cassidy R. Overview of Race and Hispanic Origin: March 2000. Washington, DC: US Census Bureau; 2001. Census 2000.
9. Immigrants’ Health Care Coverage and Access Fact Sheet. Washington, DC. Kaiser Commission on Medicaid and the Uninsured; March 2001.
10. Immigration Policy Handbook 2000. Washington, DC: National Immigration Forum;2000.
11. Jenista JA. The immigrant, refugee, or internationally adopted child. Peds in Review 2001;22:419-429.
12. Holtz T, Kachur S, MacArthur J, et al. Malaria Surveillance-United States, 1998. MMWR Morb Mortal Wkly Rep 2001;50:1-17.
13. Kain K, Harrington MA, Tennyson S, et al. Imported malaria: Prospective analysis of problems in diagnosis and management. Clinical Infectious Diseases 1998; 27:142-149.
14. Kyriacou D, Spira A, Talan D, et al. Emergency department presentation and misdiagnosis of imported falciparum malaria. Ann Emerg Med 1996;27:696-699.
15. Zuber PLF, McKenna MT, Binkin NJ, et al. Long-term risk of tuberculosis among foreign-born persons in the United States. JAMA 1997;278:304-307.
16. Kempainen R, Nelson K, Williams D, et al. Mycobacterium tuberculosis Disease in Somali Immigrants in Minnesota. Chest 2001; 119:176-180.
17. Truong D, Hedemark L. Tuberculosis among Tibetan immigrants from India and Nepal in Minnesota, 1992-1995. JAMA 1997;277: 735-738.
18. Marks G, Bai J. Effectiveness of postmigration screening in controlling tuberculosis among refugees: A historical cohort study, 1984-1998. Am J Public Health 2001;91:1797-1799.
19. Hemon A. The Question of Bruno. London: Picador; 2000.
20. Sluzki C. Migration and family conflict. Fam Process 1979;18:379-390.
21. Technical instruction for medical examination of aliens in the United States. Atlanta: Dept of Health and Human Services, CDC, Center for Prevention Services, Division of Quarantine, 1991.
22. Ivey S. Immigrant women’s health: Initial clinical assessment. West J Med 2001;174:433-437.
23. Castillo R, Waitzkin H, Escobar JU. Somatic symptoms and mental health disorders in immigrant and refugee populations. Mental Disorders in Primary Care. San Francisco: Jossey-Bass;1994:163-185.
24. Weinstein H, Dansky L, Iacopino V. Torture and war trauma survivors in primary care practice. West J Med 1996; 165:112-118.
25. Geltman PL, Radin M, Zhang Z, et al. Growth status and related medical conditions among refugee children in Massachusetts, 1995-1998. Am J Public Health 2001;91:1800-1805.
26. Avery R. Immigrant women’s health: Infectious diseases. West J Med 2001;175:208-211.
27. Loue S, ed. Handbook of Immigrant Health. New York: Plenum Press; 1998.
28. Van der veer G. The experiences of refugees. In: Chichester, UK. Counseling and therapy with refugees: psychological problems of victims of war, torture, and repression. John Wiley & Sons; 1992.
29. Rasmussen OV. Medical aspects of torture. Dan Med Bull 1990;37 (suppl):1-88.
30. McCray E, Weinbaum CM, Brader CR, et al. The epidemiology of tuberculosis in the United States. Clin Chest Med 1997;18:99-113.
31. Division of Tuberculosis Elimination, CDC. Tuberculosis morbidity among U.S.-born and foreign-born populations-United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51:101-104.
32. Recommendations for the prevention and control of tuberculosis among foreign born persons. MMWR Morb Mortal Wkly Rep 1998;47:1-29.
33. Pablos-Mendez A, Raviglione MC, Laszio A, et al. Global surveillance for antituberculosis-drug resistance, 1994-1997. N Engl J Med 1998;338:1641-1649.
34. Talbot EA, Moore M, McCray E, et al. Tuberculosis among foreign-born persons in the United States, 1993-1998. JAMA 2000; 284:2894-2900.
35. Strickland GT. Hunter’s Tropical Medicine. Philadelphia, PA; W.B. Saunders Company: 2000.
36. Sahly HM, Adams GJ, Soini H, et al. Epidemiologic differences between United States and foreign-Born tuberculosis patients in Houston, Texas. J Infect Dis 2001;183:461-468.
37. Chin DP, DeRiemer K, Small PM, et al. Differences in contributing factors to tuberculosis incidence in US-born and foreign-born persons. Am J Respir Crit Care Med 1998;158:1797-1803.
38. Fauci A, Braunwald E, Isselbacker KJ, et al, eds. Harrison’s Principles of Internal Medicine. New York; McGraw-Hill:1998.
39. Cowie RL, Sharpe JW. Extrapulmonary tuberculosis: A high frequency in absence of HIV infection. Int J Tuberc Lung Dis 1997; 1:159-162.
40. Medical Research Council Tuberculosis and Chest Diseases Unit. National survey of tuberculosis notifications in England and Wales 1978-79. BMJ 1980;281:895-898.
41. Adair R, Nwaneri M. Communicable disease in African immigrants in Minneapolis. Arch Intern Med 1999;159:83-85.
42. Ballew KA, Becker DM. Tuberculosis screening in adults who have received BCG vaccine. South Med J 1995;88:1025-1030.
43. Use of BCG Vaccines in the Control of Tuberculosis: A Joint Statement by the ACIP and the Advisory Committee for the Elimination of Tuberculosis. MMWR Morb Mortal Wkly Rep 1988;37:663-664, 669-675.
44. Targeted tuberculin testing and treatment of latent tuberculosis infection. Joint Statement, American Thoracic Society and CDC. Am J Respir Crit Care Med 2000;161:S221-S247.
45. Jerant A, Bannon M. Identification and management of tuberculosis. Am Fam Physician 2000;61:2667-2678.
46. McDermott LJ, Glassroth J, Mehta JB, et al. Tuberculosis. Part I. Dis Mon 1997;43:113-180.
47. Anonymous. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. Am J Resp Crit Care Med 2000;161(4 Pt 2):S221-S247.
48. Bruce-Chwatt LJ. History of malaria from prehistory to eradication. In: Werrnsdorfer WH, MacGregor IA, eds. Malaria: Principles and Practice of Malariology. London, UK; Churchill-Livingstone:1988.
49. Current Global Malaria Situation. WHO Expert Committee on Malaria. WHO Technical Report Series: 2000.
50. Wyler D. Malaria: Overview and update. Clin Infect Dis 1993; 16:449-458.
51. Brillman J, Quenzer R. Infection in the traveler and immigrant. In: Brillman J, Quenzer R, eds. Infectious Disease in Emergency Medicine. Philadelphia; Lippincott-Raven:1998.
52. Jelinek T, Schulte C, Behrens R, et al. Imported falciparum malaria in Europe: Sentinel surveillance data from the European Network on Surveillance of Imported Infectious Diseases. Clin Infect Dis 2002;34:572-576.
53. Campbell CC. Challenges facing antimalarial therapy in Africa. J Infect Dis 1991;163:1207-1211.
54. Greenwood, BM. Malaria chemoprophylaxis in endemic regions. Malaria: Waiting for the Vaccine. London School of Hygiene and Tropical Medicine. Target GAT, ed. London, England; 1991: 83-104. First Annual Public Health Forum
55. Olliaro P, Cattani J, Wirth D. Malaria, the submerged disease. JAMA 1996;275:230-233.
56. Miller LH, Warrell DA. Malaria. In: Warren KS, Mahmoud AAF, eds. Tropical and Geographical Medicine. 2nd ed. New York; McGraw-Hill:1990.
57. Manson-Bahr PEC, Bell DR. Manson’s Tropical Diseases. 19th ed. London, UK; Bailliere Tindall:1987.
58. White NJ, Warrell DA, Chanthavanich P, et al. Severe hypoglycemia and hyperinsulemia in falciparum malaria. N Engl J Med 1983;309:61-66.
59. Taylor TE, Molyneux ME, Wirima JJ, et al. Blood glucose levels in Malawian children before and during the administration of intravenous glucose for severe falciparum malaria. N Engl J Med 1988; 319:1040-1047.
60. Newton C, Warrell D. Neurological manifestations of falciparum malaria. Ann Neurology 1998;43:695-702.
61. Thompson M. Rapid dipstick assays for the detection of malaria. Am Fam Physician 2000;61:1640-1641.
62. Moody A, Hunt-Cook A, Gabbett E, et al. Performance of the OptiMAL antigen capture dipstick for malaria diagnosis and treatment monitoring a the Hospital for Tropical Diseases, London. Br J Haematol 2000;109:891.
63. Krogstad DJ. Malaria as a re-emerging disease. Epidemiol Rev 1996;18:77-89.
64. White NJ. The treatment of malaria. N Engl J Med 1996;335: 800-806.
65. Statistical yearbook of the Immigration and Naturalization Service, 1996. Washington, DC: Immigration and Naturalization Service, 1997.
66. Salas S, Heifetz R, Barrett-Connor E. Intestinal parasites in Central American immigrants in the United States. Arch Intern Med 1990; 150:1514-1516.
67. Molina C, Molina M, Molina J. Intestinal parasites in Southeast Asian refugees two years after immigration. West J Med 1988;149: 422-425.
68. Sarfarty M, Rosenberg Z, Siegel J, et al. Intestinal parasites in immigrant children from Central America. West J Med 1983;139: 329-331.
69. Lerman D, Barrett-Connor E, Norcross W. Intestinal parasites in asymptomatic adult Southeast Asian immigrants. J Fam Pract 1982;15:443-446
70. Flores EC, Plumb SC, McNeese MC. Intestinal parasitosis in an urban pediatric clinic population. Am J Dis Child 1983;137: 754-756.
71. Bratton R, Nesse R. Ascariasis: An infection to watch for in immigrants. Postgraduate Medicine 1993;93:171-178.
72. Liu H, Weller S. Strongyloides and other intestinal nematode infections. Infect Dis Clin North Am 1993;7:662-677.
73. Buchwald D, Lam M, Hooton TM. Prevalence of intestinal parasites and association with symptoms in Southeast Asian refugees. J Clin Pharm Ther 1975;20:271-275.
74. Nutman TB, Ottesen EA, Ieng S, et al. Eosinophilia in Southeast Asian refugees: Evaluation at a referral center. J Infect Diseases 1987;155:309-312.
75. Gill GV, Bell DR, Reid HA. Strongyloidiasis in ex-Far East prisoners of war. BMJ 1977;278:1007-1009.
76. Muennig P, Pallin D, Sell RL, et al. The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med 1999;340:773-779.
77. Berk SL, Verghese A, Alvarez S, et al. Clinical and epidemiologic features of strongyloidiasis. A prospective study in rural Tennessee. Arch Internal Medicine 1987;147:1257-1260.
78. Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature 1985; 317:489-495.
79. Lopez-Velez R, Gutierrez C. Prevalence of hepatitis B, C, and D markers in Sub-Saharan African immigrants. J Clin Gastroenterology 1997;25:650-652.
80. Torres JR, Machado IV. Special aspects of hepatitis B virus and delta virus infection in Latin America. Infect Dis Clin North Am 1994;8:13-27.
81. Catanzaro A, Moser RJ. Health status of refugees from Vietnam, Laos, and Cambodia. JAMA 1982;247:1303-1308.
82. Nelson K, Bui H, Samet J. Screening in special populations: A "Case Study" of recent Vietnamese immigrants. Am J Med 1997;102:435-440.
83. Oldfield EC, Rodier GR, Gray GC. The endemic infectious diseases of Somalis. Clin Infectious Diseases 1993;16 (Suppl 3): S132-S157.
84. Ackerman L. Health problems of refugees. J Am Board Fam Practice 1997;10:337-347
85. Kulstrunk M, Euequoz D, Dubach VC, et al. Prevalence of hepatitis B virus in Kurdish refugees. J Hepatology 1992;15: 418-419.
86. Hurie, MB, Gennis MA, Hernandez LV, et al. Prevalence of hepatitis B markers, and measles, mumps, and rubella antibodies among Jewish refugees from the former Soviet Union. JAMA 1995;273: 954-956.
87. Hurie MB, Mast EE, Davis JP. Horizontal transmission of hepatitis B virus infection to United States-born children of Hmong refugees. Pediatrics 1992;89:269-273.
88. Franks AL, Berg CJ, Kane MA, et al. Hepatitis B infection among children born in the United States to Southeast Asian refugees. New Engl J Med 1989;321:1301-1304.
89. David LG, Weber DJ, Lemon SM. Horizontal transmission of hepatitis B virus. Lancet 1989;1:889-893.
90. Walsh K, Alexander, GJ. Update on chronic viral hepatitis. Postgrad Med J 2001;77:498-505.
91. Bondesson J, Saperston A. Hepatitis. Emerg Med Clin North Am 1996;4:695-712.
92. Wolfe MS. Tropical diseases in immigrants and internationally adopted children. Med Clin North Am 1992;76:1463-1480.
93. Rosenblatt K, Weiss N, Schwartz S. Liver cancer in Asian immigrants to the United States and their descendants. Cancer Causes Control 1996;7:345-349.
94. Hoofnagle JH, diBisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med 1997;336:347-356.
95. Josefson D. Oral treatment for hepatitis B gets approval in the United States. BMJ 1998;317:1034.
96. Gardner P, Schaffner, W. Immunization of adults. N Engl J Med 1993;328:1252-1258.
97. Tyhurst L. Psychosocial first aid for refugees. Ment Health and Society 1977;4:319-343.
98. Mathers J. The gestation period of identity change. Br J Psychiatry 1974;125:473-474.
99. Grinberg L. A psychoanalytic study of migration: Its normal and pathological aspects. J Am Psychoanal Assoc 1984;32:13-38.
100. Westermeyer J, Neider J, Vang TF. Acculturation and mental health: A study of Hmong refugees at 1.5 and 3.5 years post migration. Soc Sci Med 1984;18:87-93.
101. Moore L, Boehnlein J. Post-traumatic stress disorder, depression, and somatic symptoms in U.S. Mien patients. J Nervous Mental Dis 1991;179:728-733.
102. Lin LH, Carter WB, Kleinman AM. An exploration of somatization among Asian refugees and immigrants in primary care. Am J Pub Health 1985;75:1080-1084.
103. Kinzie JD, Boehnlein JK, Leung PK et al. The prevalence of posttraumatic stress disorder and its clinical significance among Southeast Asian refugees. Am J Psych 1990;147:913-917.
104. Buchwald D, Manson SM, Brenneman DL, et al. Screening for depression among newly arrived Vietnamese refugees in primary care settings. West J Med 1996;163:341-345.
105. Kinzie JD, Boehlein JK, Leung P, et al. The high prevalence rate of PTSD and its clinical significance among Southeast Asian refugees. Am J Psychiatry 1990;147:913-197.
106. Kinzie JD, Fredrickson RH, Rath B, et al. Post-Traumatic Stress Disorder among survivors of Cambodian concentration camps. Am J Psychiatry 1984;141:5646-5650.
107. Watters C. Emerging paradigms in the mental health care of refugees. Soc Sci Med 2001;52:1709-1718.
108. Beiser M. Influences of time, ethnicity, and attachment on depression in Southeast Asian refugees. Am J Psych 1988;145:46-51.
109. Ritsner M, Ponizovsky A, Kurs R, et al. Somatization in an immigrant population in Israel: A community survey of prevalence, risk factors, and help-seeking behavior. Am J Psych 2000;157: 385-392.
110. Kraut AM. Healers and strangers. JAMA 1990;263:1807-1811.
111. Moss N, Stone M, Smith J. Child health outcomes among Central American refugees and immigrants in Belize. Soc Sci Med 1992; 2:161-167.
112. Findley S, Irigoyen M, Schulman A. Children on the move and vaccination coverage in a low-income urban Latino population. Am J Pub Health 1999;89:1728-1731.
113. Schulte JM, Maloney S, Aronson J, et al. Evaluating acceptability and completeness of overseas immunization records of internationally adopted children. Pediatrics 2002;109:E22.
114. Anonymous. Advances in global measles control and elimination: Summary of the 1997 international meeting. MMWR Morb Mortal Wkly Rep 1998;47:RR1-RR12.
115. Volti S, Mollica F. Should all children receive two measles vaccinations? Clin Pediatrics 1993;34:509-510.
116. Anonymous. Measles—United States, 2000. MMWR Morb Mortal Wkly Rep 2002; 51:120-123.
117. Anonymous. Measles—Washington, 1990. MMWR Morb Mortal Wkly Rep 1990; 39: 467-470.
118. Control and Prevention of Rubella: Evaluation and management of suspected outbreaks, measles—United States, 1989 and First 20 Weeks 1990. MMWR Morb Mortal Wkly Rep 1992;39:353-363.
119. Henry RR. Measles, Hmong, and metaphor: Culture change and illness management under conditions of immigration. Med Anthropol Quart 1999;13:32-50.
120. Rubella in pregnant women, and surveillance for congenital rubella syndrome. MMWR Morb Mortal Wkly Rep 2001;50:RR1-RR7.
