Urinary Tract Infection: Risk Stratification, Clinical Evaluation, and Evidence-Based Antibiotic Therapy
Part I: Epidemiology, Emerging Resistance Patterns, and Patient-Specific Treatment Strategies
Authors: Romolo Gaspari, MD, FACEP, Research Director, Assistant Professor, Department of Emergency Medicine, University of Massachusetts School of Medicine, Worcester, MA; and Gideon Bosker, MD, FACEP, Assistant Clinical Professor, Yale University School of Medicine, New Haven, CT.
Peer Reviewers: David S. Howes, MD, FACEP, Program Director and Chairman, Residency Program, Department of Emergency Medicine, University of Chicago Hospitals and Clinics, Associate Professor, Pritzker School of Medicine, Chicago, IL; and Ralph J. Riviello, MD, FACEP, Assistant Professor, Department of Emergency Medicine, Thomas Jefferson University, Philadelphia, PA.
In the constantly shifting landscape of drug resistance, antibiotic options, and pharmacoeconomic considerations, urinary tract infection (UTI) continues to be one of the most frequently diagnosed conditions in patients presenting to the emergency department.
It is estimated that practitioners manage 7 million new cases of cystitis in the United States each year and that, overall, UTIs account for approximately 1 million hospitalizations annually.1,2 Moreover, UTIs are the leading cause of gram-negative bacteremia in patients of all ages, and are associated with a high risk of morbidity and mortality, especially in the elderly.3 The total annual cost of treatment is in the billions of dollars.4
Among common infections managed in the outpatient setting, few conditions have treatment guidelines, antibiotic selection strategies, or diagnostic protocols that have changed or evolved as rapidly as those used for UTI. Despite a general consensus that empiric treatment of UTI in adult women requires, at the very least, mandatory coverage of Escherichia coli and other gram-negative organisms, antibiotic selection strategies—including initial choice of therapy and duration of treatment—vary widely among practitioners and institutions.
There are many reasons for inconsistencies in the current approach to UTI management among hospital-based physicians. Unfortunately, deciphering the strengths and weaknesses of recommendations issued by different authoritative sources can be problematic and confusing, especially since resistance patterns of infecting uropathogens may vary among geographic regions, and because outcome-effectiveness, medication compliance, failure rates, total-resource costs to achieve clinical cure, the risk of recurrent infection, and evolving bacterial resistance issues are not always entered into the drug selection equation.
Because no single set of guidelines is applicable to every patient or hospital practice environment, management guidelines for UTI must be "customized" for the local practice setting and, as always, clinical judgment must prevail. This means taking into account local antibiotic resistance patterns, epidemiological and infection incidence data, and patient demographic features.
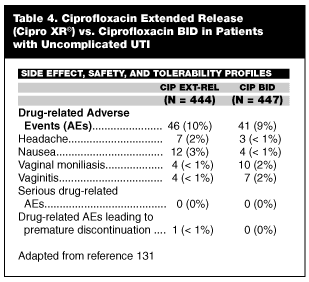
Important, new therapeutic options that have been introduced into the antimicrobial armamentarium for uncomplicated UTI offer clinicians compliance-enhancing strategies that can improve clinical outcomes. In this regard, recent introduction of extended release ciprofloxacin (Cipro XR®) has made it possible to enhance medication compliance through once-daily administration of what has been considered to be the "gold standard" antibiotic (ciprofloxacin) for uncomplicated UTI, while at the same time making available a preparation that achieves an area under the curve (AUC) that is equivalent to conventional BID ciprofloxacin and achieving a Cmax that is 40% higher than the conventional BID formulation.
From a practical clinical perspective, because of its potentially compliance-enhancing properties (once-daily dosing and a well-tolerated side effect profile), extended release delivery system, and clinically effective urine concentrations, the extended-release formulation represents a risk-management upgrade from BID ciprofloxacin; therefore, it should replace the older formulation as the clinical standard for treatment of uncomplicated UTI when indicated. A well-designed clinical trial comparing the new and conventional formulations supports this shift to the once-daily, extended-release formulation and is described in this review.
Even when these factors are considered, a number of important questions about drug selection issues for UTI still remain: 1) What is the appropriate initial, empiric choice for uncomplicated UTI? Once-daily (QD) extended-release ciprofloxacin (Cipro XR®) or trimethoprim-sulfamethoxazole (TMP-SMX)? 2) What are the specific "intensification and treatment trigger" criteria that support amplifying initial spectrum of coverage from TMP-SMX to a fluoroquinolone such as extended release ciprofloxacin? 3) How should evolving resistance of E. coli to TMP-SMX affect initial antimicrobial therapy? 4) What is the optimal duration of therapy for uncomplicated and complicated UTIs? 5) Which antibiotic currently provides correct spectrum coverage, safety, and reliability for outpatient treatment of uncomplicated UTI?
Although optimizing cure rates with so-called convenient, dose- and duration-friendly branded agents that provide appropriate and predictable coverage with a low risk of antimicrobial resistance may be perceived as costly on a drug-acquisition basis, it is important to stress the following point: Antimicrobial agents with more predictable coverage against pathogens implicated in UTI can help avoid the unnecessary costs of treatment failures, disease progression, patient re-evaluations, return visits, patient dissatisfaction, and the pharmacological reservicing costs associated with initiating a second course of antibiotics.5
In this sense, antibiotics that lower barriers to clinical cure and provide a predictable spectrum of coverage can be seen as "productivity tools" that improve efficiency of clinical care and potentially reduce the overall costs associated with inpatient and acute outpatient management of UTI.
In light of the important advances, changes, and refinements that have occurred in the area of UTI treatment during the past year, this comprehensive, state-of-the-art review presents a revised and updated set of guidelines outlining UTI epidemiology and management in outpatient and hospital-based settings. Special emphasis has been given to both epidemiological data demonstrating the importance of correct spectrum coverage with specific fluoroquinolones, such as ciprofloxacin, and the selection of initial antibiotics for patients deemed suitable for discharge.
In addition, detailed evidence-based analysis comparing ciprofloxacin to TMP-SMX is presented to guide antibiotic selection in patients with uncomplicated UTI and pyelonephritis.5 Cautionary notes about the overuse of extended spectrum fluoroquinolones are outlined, and evidence-based studies confirming ciprofloxacin’s workhorse role in hospital-based treatment of UTI is discussed. Drawing upon consensus panels, expert opinion, and clinical trials, this clinical consensus report presents antimicrobial protocols and treatment guidelines linked to, and driven by, risk-stratification criteria, evidence-based trials, and specific clinical profiles of patients presenting to the hospital with symptoms and signs suggestive of UTI.—The Editor
Introduction
Changing resistance patterns observed with common urinary pathogens have altered the empirical approach to antibiotic selection for both upper and lower UTIs. Previously, decisions regarding antimicrobial therapy have been made based on patient characteristics and the anticipated spectrum of urinary flora. However, increasing levels of resistance to beta-lactams during the past decade has decreased the utility of this drug class for treatment of UTIs. In addition, emerging resistance among E. coli species to TMP-SMX also is affecting initial drug selection choices for UTI, a change characterized by the acceptance of fluoroquinolones such as extended release ciprofloxacin as the initial agent of choice for greater than 90% of uncomplicated UTIs seen in the outpatient setting.
Epidemiology
Acute UTI is one of the most common illnesses encountered in adult women, resulting in as many as 8 million office visits per year6 and at least 100,000 hospital admissions.7,8 Although the exact frequency of UTI is not known, based on current evidence accumulated from office and hospital surveys, it is estimated that there are approximately 7 million episodes of cystitis9 and 250,000 episodes of pyelonephritis10 annually in the United States. One prospective study determined the annual incidence of cystitis to be 0.5-0.7% per person-year.11
Although many cases of uncomplicated UTI resolve with only transient, mild symptoms, studies suggest considerable morbidity and mortality are associated with all forms of UTI. Two independent studies12,13 found that asymptomatic bacteriuria in the elderly was associated with an increased mortality rate; however, this is not a universal finding.14,15 Certain patient populations, in particular those with diabetes and pregnancy, have been found to have a higher level of morbidity.16,17 Studies reviewing simple cystitis also have revealed substantial morbidity, with limited activity lasting for more than two days.18 As many as 60% of elderly patients with pyelonephritis will develop bacteremia, and 20% of these cases will result in septic shock.19 Life-threatening bacteremia as a complication of UTI also has been documented by a number of other investigators.20-24
UTIs in women vastly outnumber those in men.25 This may be related to such factors as the length of the urethra, distance of the urogenital meatus from the anus, and the antibacterial properties of prostatic fluid.26 Regardless of the reason, the fact that male UTIs are so uncommon has led many authors to classify them as complicated. This is supported by the high degree of virulence found in male UTI isolates, and the high prevalence of non-E. coli UTIs.27
Microbiology and Emerging Resistance Patterns
For many years, pathogens associated with uncomplicated UTIs remained constant, with E. coli identified as the etiologic agent in about 75-90% of infections.25 Five to 15% of uncomplicated UTIs are caused by Staphylococcus saprophyticus,28,29 with Klebsiella, Proteus, Enterococcus, and Pseudomonas species seen in much smaller percentages.30-32
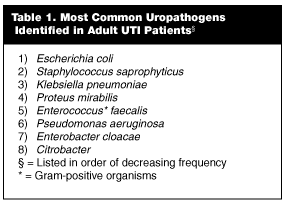
The emergence of E. coli isolates demonstrating resistance to commonly used antibiotics, especially to TMP-SMX, is changing initial drug selection patterns in patients with both uncomplicated and complicated UTIs. The most common uropathogens identified in adult patients with UTI include enteric gram-negative bacteria, with E. coli being the most common. (See Table 1.) The remainder of infections are caused by coagulase-negative Staphylococcus saprophyticus (10-20%), while Proteus mirabilis, Klebsiella, and Enterococcus account for less than 5%.3,33,34 Other aerobic gram-negative bacteria of the Enterobacteriacea family include Citrobacter, Enterobacter, Serratia, and Salmonella.35-37 Non-enteric aerobic gram-negative rods such as Pseudomonas and aerobic gram-positive cocci such as Enterococcus are less prevalent in immunocompetent hosts. (See Table 1). Group B streptococci infection is observed in neonates secondary to inoculation from a colonized mother during delivery through the vaginal canal.
Anaerobic bacteria rarely are pathogenic despite their prevalence in fecal flora. The Lactobacillus species, coagulase-negative staphylococci, and Corynebacterium are not considered clinically significant isolates in the urine of healthy children between 2 months and 2 years of age.1,38 Corynebacterium, Lactobacillus, and Streptococcus species are identified only rarely; when present in this age group, they nearly always represent contamination of the specimen rather than a true pathogen. In complicated UTI, in addition to E. coli, there is a higher prevalence of Pseudomonas, Enterobacter species, Serratia, Acinetobacter, Klebsiella, and enterococci.39 There are anecdotal reports of treatment for Gardnerella vaginalis, lactobacilli, Chlamydia trachomatis, and Ureaplasma urealyticum in pregnant women, but it is unclear whether these organisms represent true pathogens in this population.40,41 Candidal species now are emerging in greater numbers, especially in catheterized patients and those who received previous treatment for enterococcal UTIs.39
The high incidence of UTIs in the general population; the potential for complications, especially in high-risk subgroups; and the associated costs of treatment emphasize the importance of appropriate antibiotic therapy. Microbial resistance to nearly all classes of antimicrobials continues to rise despite increasing awareness and concerns worldwide. European studies have shown E. coli resistance rates to multiple antibiotics, specifically TMP-SMX, in as many as one-third of patients.42,43 Similar trends in the United States have prompted a shift to fluoroquinolones such as ciprofloxacin as preferred initial agents for empiric intravenous and/or oral therapy of UTI in both hospital and emergency department settings.44
In a cross-sectional survey of urine cultures obtained in the emergency departments of urban tertiary care centers in the United States, microbial resistance was as high as 48% to ampicillin, 25% to tetracycline, 14-28% to TMP-SMX, and 13% to nitrofurantoin.45 Similar studies have shown that the resistance to ciprofloxacin among common uropathogens, including E. coli, frequently encountered in hospital-managed UTI is as low as 1-2%.46-50
These epidemiological data have important treatment implications, since recent studies also already are demonstrating outcome differences in clinical efficacy and patient cure rates between UTI patients managed on TMP-SMX and those managed on ciprofloxacin.51 As would be expected, maintenance of predictable antimicrobial activity by ciprofloxacin against the anticipated spectrum of uropathogens has solidified the role of this antibiotic in treatment pathways for UTI among all institutional settings.
Surveillance and Sensitivity. Hospitals affiliated with managed care organizations also have been prompted to re-evaluate their initial approach to antibiotic selection for UTI. A cross-sectional survey of 4000 urine cultures obtained from women ages 18-50 in an HMO setting between 1992 and 1996 showed E. coli prevalence to be 86%, with the resistance rate to TMP-SMX increasing during this period from 9% to 18%. Recent data suggest that in some regions of the country, especially the West, Southwest, and in most major urban centers, the resistance rate to TMP-SMX has risen to as high as 35%.5,42,43,52-55 The overall resistance to multiple groups of antimicrobials, including the penicillins, cephalosporins, and sulfa drugs, doubled from 8% to 16%.56 In pregnant patients, E. coli resistance to ampicillin, which at one time was a drug of choice for UTI in this population, is now about 20-30%.41
Fortunately, one class of antimicrobials to which sensitivity rates have remained consistently high is the fluoroquinolone group, of which ciprofloxacin is the most frequently used in the adult population. A two-tiered study from 1989 to 1991 and 1996 to 1997 at an urban sexually transmitted disease clinic evaluated young, sexually active females diagnosed with a UTI and found E. coli resistance rates to ampicillin, cephalosporins, or tetracycline in as many as 25% of patients. There was very little change in the low prevalence of organisms resistant to fluoroquinolones.57
Additional studies at student health clinics in California during a five-year period demonstrated significant increases in the resistance of E. coli to ampicillin (30-45%), tetracycline (29-40%), and TMP-SMX (15-32%), with resistance to fluoroquinolones in fewer than 5% of organisms.34 In a recent analysis of young women with uncomplicated pyelonephritis, E. coli was isolated in more than 90% of cultures and was resistant to TMP-SMX in 18%, compared with a 0.4% resistance to ciprofloxacin. A significant variance in resistance patterns existed in different geographic regions, with resistance to TMP-SMX as high as 35% on the West Coast of the United States as opposed to 14% in the Midwest and 7% on the East Coast.51 One caveat regarding bacterial resistance is that in vitro sensitivity results may not correlate with clinical cure rates and in vivo sensitivity. Eradication of a uropathogen depends on the concentration of antibiotics in the urine as opposed to serum, which may be higher than the levels used in in vitro studies.39
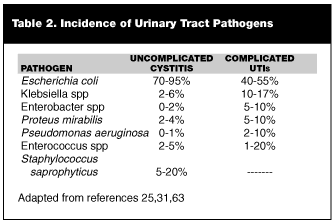
Recent studies have noted a subtle shift in etiologic agents associated with UTIs. A survey of all UTI pathogens in 1997 found the top four isolates to be E. coli (48.6%), Enterococcus spp (13.7%), Klebsiella spp (12.0%), and Pseudomonas aeruginosa (6.2%).58 More current data from 1998 support these trends.59 This shift in pathogens may be related to factors such as bladder catheterization or antibiotic use. Another study reported a similar mix of uropathogens in catheter-associated UTIs.60 Not surprisingly, bacterial isolates found in complicated UTIs follow a similar pattern.61,62 (See Table 2.)
Although there are fewer data on patients with pyelonephritis, recently published surveys indicate a similar mix of pathogens, with about 90% of patients with pyelonephritis manifesting infection with E. coli.64,65 Other isolates included those found in lower UTIs. Urethritis usually is caused by Chlamydia trachomatis, Neisseria gonorrhoeae, or herpes simplex virus.
Escherichia coli (E. coli) Resistance. The sensitivity of a urinary tract pathogen to a specific antimicrobial agent is defined by measuring the bacteria’s ability to grow in the presence of that antibiotic. In the case of E. coli, if the strain under evaluation can grow in media containing 2 mcg/mL or greater (mean inhibitory concentration or MIC) of the antibiotic, the strain is considered resistant to that antibiotic. Potential confusion arises when the reported resistance levels are related to the blood concentration of antibiotic and not the urine concentration. As a rule, antibiotics used for UTIs are concentrated in the urine and have higher urine levels than blood levels. Therefore, isolates that are reported resistant to an antibiotic by laboratory testing actually may be eradicated by the antibiotic in the in vivo environment. This concept is clinically relevant and has been demonstrated in a number of studies.28,66,67
Due to the consistent, relatively predictable spectrum of pathogens encountered in UTIs, empiric therapy represents an appropriate strategy for the majority of patients, whether they present in the outpatient, emergency department, or in-hospital setting. Historically, empiric therapy using any one of a wide range of agents has proven clinically successful for UTI. Until recently, high cure rates could be expected because a predictable group of urinary pathogens have manifested a low degree of resistance to most antibiotics selected on an empiric basis. However, a number of recent studies have highlighted evolving changes in antimicrobial resistance patterns to E. coli. In particular, clinically and microbiologically significant changes in resistance to TMP-SMX among E. coli species, and in a small percentage of cases, to fluoroquinolones have been reported in Europe68,69 and the United States.30-32,59,70
Resistance and Implications for Antibiotic Therapy—Fluoroquinolones Emerge as Initial Agents of Choice. Evolving changes in drug resistance dramatically have altered the approach to empiric therapy of UTI. Although beta-lactams, sulfa-based antibiotics, and fluoroquinolones each have their place in the treatment of UTI, their roles are changing, with fluoroquinolones emerging as initial agents of choice, even for uncomplicated UTI. Penicillin-based antibiotics once were a mainstay of UTI treatment, but current resistance rates among E. coli (approaching 40% in many regions) have limited their effectiveness.30,71,72 Although E. coli resistance to fluoroquinolones in the United States has not reached the levels encountered with other antibiotics,25,28,72 the level of resistance in other countries is alarming.73,74 In the United States, however, increasing E. coli resistance to TMP-SMX has been accompanied by a paradigm shift in the initial treatment of choice for UTI (please see below).31,75-78
The level of E. coli resistance to TMP-SMX has more than doubled during the past 12 years and now exceeds 25% in some areas of the country.79-81 One group of investigators examined a cross-section of urinary isolates from 1992 to 1996 and found an increase of TMP-SMX resistance from 9% to more than 18%.72 Subsequent, larger studies have shown similar results.79-81 Resistance rates in the United States vary from region to region, and knowledge of local resistance rates are important factors when determining initial antibiotic therapy.31
Most experts and national association panels concur that sequential selection strategies for antibiotic therapy in UTI, to a significant degree, should depend on the degree of E. coli resistance to TMP-SMX in a particular community. In this regard, the Infectious Disease Society of America (IDSA) recommends that alternative antibiotics (i.e., agents other than TMP-SMX) should be used as first-line therapy in areas of the country where TMP-SMX resistance is greater than 10-20%.78 More specifically, the clinical outcome and pharmacoeconomic implications of fluoroquinolones vs. TMP-SMX therapy have been linked to a 20% E. coli drug resistance cut-off point.
With this antibiotic preference issue in mind, two published studies have examined the effect of E. coli resistance rates on patient outcomes75 and economic parameters.82 One study concluded that the clinical effectiveness of fluoroquinolones such as ciprofloxacin was superior to TMP-SMX when more than 10% of the E. coli isolates were resistant to TMP-SMX.75 At a 20% resistance rate, nitrofurantoin also was superior to TMP-SMX. Another study, using a cost-analytical model, approached the issue of antimicrobial selection from a different angle. Performing a cost analysis of first-line UTI antibiotic options—examining the desirability of one agent vs. another through the prism of increasing antibiotic resistance rates—these investigators found progressive cost savings to the community when a fluoroquinolone was substituted for TMP-SMX as initial agents of choice in areas characterized by a 20% or greater resistance rate among E. coli to TMP-SMX.82 Although authorities identify different resistance rate breakpoints that would favor a shift to a fluoroquinolone as first-line therapy, there is general agreement that the greater the resistance rate, the greater the clinical and pharmacoeconomic benefits to fluoroquinolone use.
It should be stressed that the fluoroquinolones are not immune to the selective pressures causing antibiotic resistance in UTI isolates. Studies in some foreign countries, where there has been heavy use of this class of antibiotics, have shown increasing rates of resistance. A multi-center study found E. coli resistance to ciprofloxacin in 36% and 20% of urinary isolates from Portugal and Spain, respectively.30 However, most studies of urinary isolates in the United States show only a 1-4% resistance rate to fluoroquinolones.31,59
Multi-drug resistant uropathogens are becoming increasingly common across the United States. One retrospective study found that 37% of UTI isolates from emergency department patients were multi-drug resistant.32 A larger national study of inpatients as well as outpatients looked at almost 39,000 urinary isolates from patients with UTIs and found the number of multi-drug resistant isolates to be 7.1%. Among the resistant strains, 98% were resistant to ampicillin and 93% were resistant to TMP-SMX. The resistance to ciprofloxacin and nitrofurantoin was 39% and 8%, respectively.79
Urinary Tract Infections in Women—Principles of Management
Asymptomatic Bacteriuria. Asymptomatic bacteriuria is a well-documented entity that affects women of all ages. A number of large-scale population studies have shown the prevalence of asymptomatic bacteriuria to be directly related to age, with a 1-2% rate in young women, 6-10% in women older than age 60,83 and 15-20% in women ages 65 and older.15,84,85 These rates only represent a "snapshot in time," inasmuch as studies have demonstrated a "turnover" of bacteriuria from positive to negative and back again during sequential six-month urine cultures.86,87
As a rule, asymptomatic bacteriuria (ASB) should not treated in most patients, since multiple studies have shown that antibiotic therapy does not make a significant impact on long-term outcomes in an otherwise healthy adult population.88 A recent prospective study of asymptomatic bacteriuria in sexually active young women found prevalence rates of approximately 5%, with 8% of those women developing symptomatic UTI within one week.89 Specific groups benefiting from antibiotic treatment include pregnant women, neutropenic patients, patients with abnormal renal function, renal transplant recipients in the early post-transplantation period, and men and women planning to undergo urologic procedures.3,89
Infants with ASB represent a low-risk population for the development of UTIs, with a tendency toward spontaneous abacteriuria within a few months, and do not generally require antibiotic treatment.90 School-age children usually are left untreated; however, patients with underlying voiding disorders should be referred appropriately for further evaluation and treatment.
Pregnant women with ASB should be treated with a three- to seven-day course of antibiotics, followed by a subsequent culture to ensure sterilization of urine. Despite increasing resistance rates to ampicillin, amoxicillin and cephalosporins remain a first-line choice in these patients. Ceftriaxone is the preferred agent in pregnant women. Nitrofurantoin is becoming a first-line drug, because it is efficacious, inexpensive, and well-tolerated. The only contraindication to using this drug is in patients with G6PD deficiency, in whom hemolysis can occur. TMP-SMX remains a first-line agent in areas of low resistance, but should be avoided in the first and third trimesters secondary to possible teratogenic effects and the risk of kernicterus from competitive binding of TMP-SMX to bilirubin binding sites. At this time there is no clear evidence to support a single-dose regimen over a typical three- to seven-day course.40,91 A properly sized, randomly controlled trial is recommended for comparison of these regimens, as a single dose has lower cost, fewer adverse effects, and increased compliance compared with longer treatment regimens.91
Initial evidence in the elderly population had suggested an increased risk of morbidity and mortality in patients with ASB. More recent studies have challenged these reports, but have failed to identify a connection between ASB and an increase in long-term sequelae such as hypertension or end-stage renal disease. Up to 40% of the elderly will have ASB at some time. Aggressive screening and treatment have little effect on decreasing symptomatic or clinically significant infection and associated complications.3 Catheterized patients, including those with neurologic disorders or spinal cord injuries, rarely require aggressive work-up and treatment unless symptoms intervene.93 Interestingly, a recent study of catheterized patients found that catheter-associated UTIs are rarely symptomatic and infrequently cause bacteremia (< 1%). No significant differences were noted between symptomatic and asymptomatic bacteriuria groups with regard to signs and symptoms commonly associated with infection (i.e., fever, dysuria, urgency, or flank pain) or leukocytosis.94 Investigations have noted that both groups are a major reservoir for antibiotic resistant organisms in the hospital setting.
The long-term consequences of asymptomatic bacteriuria have not been fully elucidated, but this condition may have the potential to cause symptomatic UTIs and/or sepsis in a small minority of patients, although such sequelae have not been uniformly substantiated. In addition, the potential for persistent bacteriuria and recurrent UTI to decrease survival continues to be debated, with some investigators finding an association12,13 while others have not.14,15 A recent, well-conducted analysis of patients with asymptomatic bacteriuria failed to show an increase in mortality when comorbid factors were accounted for.95 In summary, most experts currently do not recommend treatment for asymptomatic bacteriuria, except in the high-risk patient populations identified above.83,96
Uncomplicated Lower Urinary Tract Infection. The majority of uncomplicated lower UTIs occur in patients who have no functional or anatomical abnormality of their urinary system. Lower UTIs can be categorized into two distinct categories: cystitis and urethritis. In the emergency department, it may be difficult to distinguish between uncomplicated and complicated cystitis. As a rule, however, the clinician will be able to distinguish between cystitis and urethritis on the basis of history and physical exam. Symptoms of urethritis overlap with cystitis, but urethritis usually has a more gradual onset with milder symptoms and little to no urgency or frequency. Associated vaginal discharge or lesions may make the diagnosis more apparent. It usually is reasonable to assume that a young, non-pregnant female with acute onset of dysuria and frequency has a simple cystitis if she has not had any instrumentation or recent antibiotic therapy.
Certain patient populations are at an increased risk for developing a UTI. One group has determined that recent sexual intercourse, diaphragm use, and a history of previous UTIs are independent risk factors for developing a UTI.11 Although the study population was limited to college age women, other studies that included older women have demonstrated similar risk factors.97 However, these risk factors are not universally agreed upon and many patients with a confirmed diagnosis of UTI will cite no risk factors.
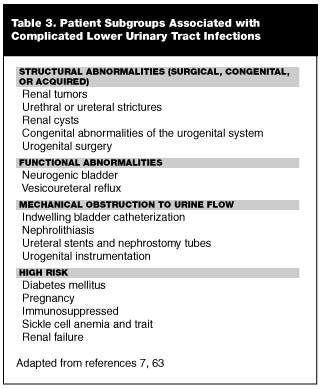
Complicated Lower Urinary Tract Infections and High Risk Patients. Complicated UTIs typically occur in patients who have an underlying urinary tract abnormality causing obstruction. The abnormality may be a physical obstruction (i.e., kidney stone or bladder catheter), or it may be a functional abnormality (i.e., neurogenic bladder or vesicoureteral reflux). Infections in patients with such disease states as diabetes or renal failure, as well as renal transplantation, also fall into the category of so-called complicated UTI. (See Table 3.) Complicated UTIs encompass patients with a wide variety of syndromes and risk factors that increase the likelihood of poor outcomes. Moreover, studies aimed toward evaluating etiological agents in complicated infections demonstrate a broad range of microbial pathogens, of which E. coli is the most common.63
An associated concept is the high-risk patient with UTI, which would include individuals who are pregnant, immunosuppressed, or those who have an indwelling catheter. High-risk patients may or may not have a urogenital abnormality, but the potential sequelae of UTI in these subgroups may overlap with those encountered in patients with complicated infections; as a result, some authors use the terms interchangeably. From a clinical perspective, the important concept is that both patients who have a complicated UTI and those deemed at high risk require more prudent management, which includes a longer duration of therapy and closer follow up.
Upper Urinary Tract Infection. Upper UTI generally refers to pyelonephritis and its potential complications, including perinephric abscess. Many studies have confirmed that pyelonephritis occurs through ascending infection from the bladder.98,99 It is thought that fecal organisms inoculate the urethra, spread into the bladder, and subsequently ascend the ureters to the kidney parenchyma. The incidence of pyelonephritis is much lower then that of cystitis, although the morbidity and mortality are much greater.25 Acute pyelonephritis can progress to chronic pyelonephritis or perinephric abscess depending on host factors such as immune status and obstruction.100
Symptoms of pyelonephritis can vary from dysuria to fulminant urosepsis. Only 20-30% of patients with isolated dysuria actually will have subclinical pyelonephritis.101-103 Patients with subclinical pyelonephritis present with symptoms characteristic of cystitis, but infection is located in the kidney. Subclinical pyelonephritis is impossible to differentiate from cystitis without complicated localization techniques that mostly are used in research studies. Most patients with symptomatic pyelonephritis will present with flank pain, nausea, vomiting, fever, and costovertebral angle tenderness.104

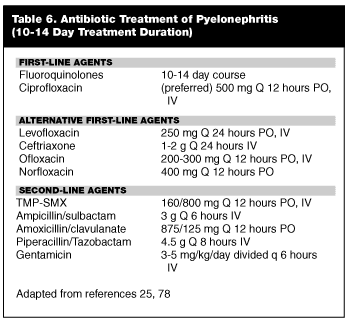
Diagnostic Strategies in Urinary Tract Infection: Urinalysis, Culture, and Imaging Studies
The majority of patients with UTI present with unambiguous symptoms and signs suggestive of this disease process, and therefore, present few diagnostic challenges for the astute clinician. However, a minority of infections, especially those encountered in the elderly or immunocompromised individual, may be more clinically challenging. For example, most clinicians would have little difficulty making the diagnosis of UTI in a young woman presenting with one day of dysuria and frequency, whereas diagnostic challenges are likely to surface when UTI causes obtundation in an elderly male. Because a number of modalities are available for diagnostic evaluation, the practitioner must determine which laboratory tests and/or imaging modalities are appropriate and cost-effective in individuals presenting with symptoms suggestive of UTI.
Diagnostic Approach. Clinical experience suggests that most uncomplicated lower UTIs encountered in the outpatient setting can be diagnosed (and cured) without the use of urine cultures.75 Accordingly, patients who present with symptoms consistent with cystitis or urethritis should undergo a history, physical exam, and urinalysis. The use of dipstick urinalysis and/or microscopic urinalysis provides a diagnostic yield that is sufficiently specific and sensitive to establish the diagnosis of uncomplicated UTI.105 Due to the increased morbidity and mortality associated with pyelonephritis and UTI in high-risk patients, a urine culture still is recommended for the work-up in this patient subgroup.
Urinalysis. A urinalysis continues to be the "workhorse" laboratory evaluation for establishing the diagnosis of UTI in a broad range of patients; therefore, it is the principal modality employed for the work-up of UTI. The primary utility of a urinalysis is to examine for and document the presence of pyuria, hematuria, nitrates, leukocyte esterase, and bacteria. Although semi-quantitative dipstick and microscopic urinalysis are widely used and have been extensively reviewed in the literature, studies are conflicting as to their accuracy.105-110
The presence of red or white cells in the urine can help differentiate the location of the infection. Pyuria is present in almost all patients with urethritis, cystitis, and pyelonephritis. The laboratory/clinical definition of pyuria (number of white cells per high-powered field [hpf]) will affect its sensitivity and specificity for establishing the diagnosis of UTI. One group found that the presence of greater than 5 WBCs per hpf was 85% sensitive for UTI, whereas other authors111 have reported that greater than 10 WBCs/hpf was a more reliable breakpoint for making the diagnosis.107 The presence or absence of pyuria should be interpreted within the context of other findings in the urinalysis. Hematuria can be present with cystitis and pyelonephritis but rarely is seen in urethritis.25 It can be diagnosed semi-quantitatively with a urine dipstick or quantitatively on a microscopic urinalysis. Studies looking at dipstick hematuria have found a sensitivity and specificity of 44% and 88%, respectively.112 Microscopic analysis of the urine also may demonstrate red cell casts that are indicative of upper tract disease.
Several unique esterases produced by neutrophils in the urine form the basis of one of the screening tests for UTI. The leukocyte esterase can be detected rapidly using a urine dipstick, which appears to provide a reliable method for detecting pyuria. Studies have shown that the leukocyte esterase has a sensitivity in the range of 74-96% and a specificity of about 94-98%,108,113,114 although this may not distinguish the presence of pyuria with this degree of accuracy in clinical practice.112,115
The nitrite test on a dipstick urinalysis is a rapid screening test for bacteriuria. It has been found to be 39% sensitive and 93% specific for bacteria in the urine in both prospective and retrospective studies.105,107,112 One investigation combined nitrate results with the presence of microscopic bacteriuria and/or pyuria (> 10 WBCs per hpf) and found a sensitivity and specificity in the range of 71-95% and 54-86%, respectively.107 Other studies have found that the accuracy of this test can be affected by a low level of infection20 or the type of infecting microorganism.116
Urine Culture. Bacteriuria is considered by most clinicians to be the definitive marker of UTI. Studies conducted in the 1950s found that 105 colony forming units (cfu) per milliliter was indicative of a UTI.117,118 However, more recent studies suggest that this level of bacteriuria may miss a large group of UTIs, and support the concept that lower levels (102-104 cfu) should be considered positive.7,119 In one provocative study, patients provided urine samples when a diagnosis of UTI was suspected, but they were not treated for two days after onset of symptoms. A repeat urine culture was obtained two days later at which time empiric treatment was started. Interestingly, urine cultures cleared spontaneously in only 5% of the patients who had a low colony count initially, while 48% now had a colony count of 105 or more.120
It should be emphasized that lower levels of bacteriuria have been shown to predict UTIs in a variety of settings. Levels greater than 102 have shown a sensitivity of 95% and a specificity of 85% for the diagnosis of cystitis in women.121 Male patients with urine samples growing greater than 103 cfu/mL are considered positive for UTI.26 All patients with pyelonephritis have been found to have a higher level of bacteriuria, with cultures almost uniformly growing at levels greater than 104 cfu/mL.117,122 In summary, studies suggest that antibiotic therapy should be considered for any patient with symptoms of a UTI and a culture positive for 103 cfu/mL or greater of a urinary tract pathogen.
Urine Collection. The method by which urine is collected has received little attention in the scientific literature. The most commonly recommended collection technique for women is either a mid-stream clean-catch urine sample, or an in-and-out catheterized specimen. Despite the effort that goes into instruction for a mid-stream clean-catch urine sample, contamination is a frequent problem.123 There is little rigorous scientific research that supports a mid-stream clean-catch urine sample as the standard for urine collection. In one study, urine samples randomly were collected by one of two techniques. One group received instructions on cleaning and technique for obtaining a mid-stream clean-catch urine specimen. The other group did not clean, and no other instructions were given. There was no difference in contamination between the groups. Studies in men revealed similar results.124,125
In-and-out sterile catheterization is the most reliable method to obtain a urine sample from women. This procedure has the lowest chance of contamination from vaginal or perineal flora and has a low risk of complications. There is a risk of inducing infection in 1-3% of patients.126 Catheterizing a male to get a urine sample is not recommended, as any urine sample usually is clean. If the situation demands obtaining a urine sample from a male who cannot cooperate, a catheterized specimen is recommended, but a clean "condom" catheter may as useful as bladder catheterization.127
Imaging Techniques. Radiographic imaging has no role in the initial work up of most UTIs. Some specific imaging modalities may have utility in identifying upper UTIs or their complications. Ultrasound is relatively poor at identifying infectious conditions of the kidney other than perinephric abscess, infected hydronephrosis, or emphysematous pyelonephritis; fortunately, the conditions are rare.128 CAT scan has been found to be better for visualizing all infectious conditions of the kidney and has the advantage of identifying alternative, non-infectious conditions.129,130 The few categories of patients with UTI who should be considered for imaging are patients with recurrent illness or patients who are not improving despite therapy.
References
1. Lifshitz E, Kramer L. Outpatient urine culture: Does collection technique matter? Arch Intern Med 2000;160:2537-2540.
2. Roberts JA. Management of pyelonephritis and upper urinary tract infections. Urol Clin North Am 1999;26:753-763.
3. Orenstein R, Wong ES. Urinary tract infections in adults. Am Family Phys 1999;59:1225-1234.
4. Saint S, Scholes D, Fihn SD, et al. The effectiveness of a clinical practice guideline for the management of presumed uncomplicated urinary tract infection in women. Am J Med 1999;106:636-641.
5. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women. JAMA 2000;283:12.
6. The National Kidney and Urologic Diseases Advisory Board 1990 long-range plan—window on the 21st century. Bethesda, MD: National Institutes of Health; 1990.
7. Johnson JR, Stamm WE. Urinary tract infections in women: Diagnosis and treatment. Ann Intern Med 1989;111:906-917.
8. Patton JP, Nash DB, Abrutyn E. Urinary tract infection: Economic considerations. Med Clin North Am 1991;75:495-513.
9. Schappert SM. National Ambulatory Medical Care Survey: 1992 summary. Adv Data 1994;253:1-20.
10. Stamm WE, Hooton TM, Johnson JR, et al. Urinary tract infections: From pathogenesis to treatment. J Infect Dis 1989;159:400-406.
11. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 1996;335:468-474.
12. Sourander LB, Kasanen A. A 5-year follow-up of bacteriuria in the aged. Gerontol Clin (Basel) 1972;14:274-281.
13. Dontas AS, Kasviki-Charvati P, Papanayiotou PC, et al. Bacteriuria and survival in old age. N Engl J Med 1981;304:939-943.
14. Nicolle LE, Henderson E, Bjornson J, et al. The association of bacteriuria with resident characteristics and survival in elderly institutionalized men. Ann Intern Med 1987;106:682-686.
15. Nordenstam GR, Brandberg CA, Oden AS, et al. Bacteriuria and mortality in an elderly population. N Engl J Med 1986;314: 1152-1156.
16. Geerlings SE, Stolk RP, Camps MJ, et al. Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Arch Intern Med 2001;161:1421-1427.
17. McDermott S, Daguise V, Mann H, et al. Perinatal risk for mortality and mental retardation associated with maternal urinary-tract infections. J Fam Pract 2001;50:433-437.
18. Foxman B, Frerichs RR. Epidemiology of urinary tract infection: I. Diaphragm use and sexual intercourse. Am J Public Health 1985; 75:1308-1313.
19. Gleckman RA, Bradley PJ, Roth RM, et al. Bacteremic urosepsis: A phenomenon unique to elderly women. J Urol 1985;133:174-175.
20. Freid MA, Vosti KL. The importance of underlying disease in patients with gram-negative bacteremia. Arch Intern Med 1968; 121:418-423.
21. Roberts FJ. A review of positive blood cultures: Identification and source of microorganisms and patterns of sensitivity to antibiotics. Rev Infect Dis 1980;2:329-339.
22. Krieger JN, Kaiser DL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis 1983;148: 57-62.
23. Kreger BE, Craven DE, Carling PC, et al. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med 1980;68:332-343.
24. Bryan CS, Reynolds KL. Hospital-acquired bacteremic urinary tract infection: Epidemiology and outcome. J Urol 1984;132:494-498.
25. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 1997;11:551-581.
26. Lipsky BA. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med 1989;110: 138-150.
27. Ulleryd P, Lincoln K, Scheutz F, et al. Virulence characteristics of Escherichia coli in relation to host response in men with symptomatic urinary tract infection. Clin Infect Dis 1994;18:579-584.
28. McCarty JM, Richard G, Huck W, et al. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Ciprofloxacin Urinary Tract Infection Group. Am J Med 1999;106: 292-299.
29. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 1999;281:736-738.
30. Kahlmeter G. The ECO*SENS Project: A prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens-interim report. J Antimicrob Chemother 2000;46(Suppl A):15-22.
31. Gupta K, Sahm DF, Mayfield D, et al. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: A nationwide analysis. Clin Infect Dis 2001; 33:89-94.
32. Wright SW, Wrenn KD, Haynes M, et al. Prevalence and risk factors for multidrug resistant uropathogens in ED patients. Am J Emerg Med 2000;18:143-146.
33. Lutters M, Vogt N. Antibiotics duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database of Systematic Rev 2000;2.
34. Anderson R. Management of lower urinary tract infections and cystitis. Urol Clin North Am 1999;26:729-735.
35. Steele RW. The epidemiology and clinical presentation of urinary tract infections in children 2 years of age through adolescence. Pediatr Ann 1999;28:653-658.
36. American Academy of Pediatrics. Practice parameter: The diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 1999;103:843-852.
37. Jacobson SH, Eklof O, Eriksson CG, et al. Development of hypertension and uremia after pyelonephritis in childhood: 27 year follow up. BMJ 1989;299:703-706.
38. Shaw KN, Gorelick MH. Urinary tract infection in the pediatric patient. Pediatr Clin North Am 1999;46:1111-1124.
39. Wood CA, Abrutyn E. Urinary tract infection in older adults. Clin Geriatr Med 1998;14:267-283.
40. Connoly AM, Thorp JM. Urinary tract infections in pregnancy. Urol Clin North Am 1999;26:779-787.
41. Delzell JE, Lefevre ML. Urinary tract infection in pregnancy. Am Fam Phys 2000;61:713-721.
42. Newell A, Riley P, Rogers M. Resistance patterns of urinary tract infections diagnosed in a genitourinary medicine clinic. Int J STD AIDS 2000;11:499-500.
43. Baerheiy A, Digranes A, Hunskar S. Are resistance patterns published by microbiological laboratories valid for general practice? APMIS 1999;107:676-680.
44. O’Donnell JA, Gelone SP. Antibacterial therapy: Fluoroquinolones. Infect Dis Clin North Am 2000;14:489-513,xi.
45. Marco CA, Parker K. Antimicrobial resistance among organisms causing urinary tract infections [letter]. Acad Emerg Med 1997; 4:159-160.
46. Mombelli G, Pezzoli R, Pinoja-Lutz G, et al. Oral vs. intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections. A prospective randomized clinical trial. Arch Intern Med 1999;159:53-8.
47. Flanagan PG, Davies EA, Stout RW. A comparison of single-dose vs. conventional-dose antibiotic treatment of bacteriuria in elderly women. Age Aging 1991;20:206-211.
48. Wiseman LR, Balfour JA. Ciprofloxacin, a review of its pharmacological profile and therapeutic use in the elderly. Drugs & Aging 1994;4:145-173.
49. Li-McLeod J, Cislo P, Gomolin IH. Cost analysis of ciprofloxacin oral suspension vs. trimethoprim/sulfamethoxazole oral suspension for treatment of acute urinary tract infections in elderly women. Presented at the American Society of Consultant Pharmacists Annual Meeting, Nov. 1-4, 2000; Boston, MA. Abstract #3.
50. Stapleton A, Stamm WE. Prevention of urinary tract infection. Infect Dis Clin North Am 1997;11:719-733.
51. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women. JAMA 2000;283:1583-1590.
52. Blaine WB, Yu W, Summe JP. Epidemiology of hospitalization of elderly Medicare patients for urinary tract infections, 1991-1996, Abstract L-87, Presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept. 15-18, 1996; San Diego, CA.
53. Patton JP, Nash DB, Abrutyn E. Urinary tract infection: Economic considerations. Med Clin North Am 1991;75:495-513.
54. Haley RW, Culver DH, White JW. The nationwide nosocomial infection rate: A new need for vital statistics. Am J Epidemiol 1985; 121:159-167.
55. Boscia JA, Kobasa WD, Knight RA, et al. Epidemiology of bacteriuria in an elderly population. Am J Med 1986;80:208-214.
56. Simon D, Trenholme G. Antibiotic selection for patients with septic shock. Crit Care Clin 2000;16:215-231.
57. Gupta K, Hooton TM, Wobbe CL, et al. The prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in young women. Int J Antimicrob Agents 1999;114:305-308.
58. Jones RN, Kugler KC, Pfaller MA, et al. Characteristics of pathogens causing urinary tract infections in hospitals in North America: Results from the SENTRY Antimicrobial Surveillance Program, 1997. Diagn Microbiol Infect Dis 1999;35:55-63.
59. Mathai D, Jones RN, Pfaller MA. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: A report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn Microbiol Infect Dis 2001;40:129-136.
60. Harding GK, Nicolle LE, Ronald AR, et al. How long should catheter-acquired urinary tract infection in women be treated? A randomized controlled study. Ann Intern Med 1991;114:713-719.
61. Nicolle LE, Louie TJ, Dubois J, et al. Treatment of complicated urinary tract infections with lomefloxacin compared with that with trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother 1994;38:1368-1373.
62. Cox CE, Holloway WJ, Geckler RW. A multicenter comparative study of meropenem and imipenem/cilastatin in the treatment of complicated urinary tract infections in hospitalized patients. Clin Infect Dis 1995;21:86-92.
63. Nicolle LE. A practical guide to the management of complicated urinary tract infection. Drugs 1997;53:583-592.
64. Johnson JR, Lyons MF 2nd, Pearce W, et al. Therapy for women hospitalized with acute pyelonephritis: A randomized trial of ampicillin versus trimethoprim-sulfamethoxazole for 14 days. J Infect Dis 1991;163:325-330.
65. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA 2000;283:1583-1590.
66. Nicolle LE, Hoepelman AI, Floor M, et al. Comparison of three days’ therapy with cefcanel or amoxicillin for the treatment of acute uncomplicated urinary tract infection. Scand J Infect Dis 1993;25: 631-637.
67. Masterton RG, Bochsler JA. High-dosage co-amoxiclav in a single dose versus 7 days of co-trimoxazole as treatment of uncomplicated lower urinary tract infection in women. J Antimicrob Chemother 1995;35:129-137.
68. Goettsch W, van Pelt W, Nagelkerke N, et al. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in the netherlands. J Antimicrob Chemother 2000;46:223-228.
69. Sotto A, De Boever CM, Fabbro-Peray P, et al. Risk factors for antibiotic-resistant Escherichia coli isolated from hospitalized patients with urinary tract infections: A prospective study. J Clin Microbiol 2001;39:438-444.
70. Steinke DT, Seaton RA, Phillips G, et al. Prior trimethoprim use and trimethoprim-resistant urinary tract infection: A nested case-control study with multivariate analysis for other risk factors. J Antimicrob Chemother 2001;47:781-787.
71. Dyer IE, Sankary TM, Dawson JA. Antibiotic resistance in bacterial urinary tract infections, 1991 to 1997. West J Med 1998;169: 265-268.
72. Gupta K, Stamm WE. Pathogenesis and management of recurrent urinary tract infections in women. World J Urol 1999;17:415-420.
73. Iqbal J, Rahman M, Kabir MS. Increasing ciprofloxacin resistance among prevalent urinary tract bacterial isolates in Bangladesh. Jpn J Med Sci Biol 1997;50:241-250.
74. Garcia-Rodriguez JA. Bacteriological comparison of cefixime in patients with noncomplicated urinary tract infection in Spain. Preliminary results. Chemotherapy 1998;44(Suppl 1):28-30.
75. Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med 2001;135:41-50.
76. Stamm WE. An epidemic of urinary tract infections? N Engl J Med 2001;345:1055-1057.
77. Hooton TM, Levy SB. Antimicrobial resistance: A plan of action for community practice. Am Fam Physician 2001;63:1087-1098.
78. Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999;29:745-758.
79. Sahm DF, Thornsberry C, Mayfield DC, et al. Multidrug-resistant urinary tract isolates of Escherichia coli: Prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother 2001;45:1402-1406.
80. Zhanel GG, Karlowsky JA, Low DE, et al. Antibiotic resistance in respiratory tract isolates of Haemophilus influenzae and Moraxella catarrhalis collected from across Canada in 1997-1998. J Antimicrob Chemother 2000;45:655-662.
81. Newell A, Riley P, Rodgers M. Resistance patterns of urinary tract infections diagnosed in a genitourinary medicine clinic. Int J STD AIDS 2000;11:499-500.
82. Le TP, Miller LG. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: A decision and cost analysis. Clin Infect Dis 2001;33:615-621.
83. Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997;11:647-662.
84. Boscia JA, Kobasa WD, Knight RA, et al. Epidemiology of bacteriuria in an elderly ambulatory population. Am J Med 1986;80: 208-214.
85. Kasviki-Charvati P, Drolette-Kefakis B, Papanayiotou PC, et al. Turnover of bacteriuria in old age. Age Ageing 1982;11:169-174.
86. Abrutyn E, Mossey J, Levison M, et al. Epidemiology of asymptomatic bacteriuria in elderly women. J Am Geriatr Soc 1991;39: 388-393.
87. Boscia JA, Abrutyn E, Kaye D. Asymptomatic bacteriuria in elderly persons: treat or do not treat? Ann Intern Med 1987;106:764-766.
88. Nicolle LE. Asymptomatic bacteriuria-important or not? N Engl J Med 2000;343:1037-1039.
89. Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000;343:992-997.
90. Rushton HG. Urinary tract infections in children: Epidemiology, evaluation and management. Pediatr Urol 1997;44:1133-1167.
91. Villar J, Lydon-Rochelle MT, Gülmezoglu AM, et al. Duration of treatment for asymptomatic bacteriuria during pregnancy (Cochrane Review). In: The Cochrane Library, Issue 3, 2000. Oxford: Update Software.
92. Vazques JC, Villar J. Treatments for asymptomatic urinary tract infections during pregnancy (Cochrane Review). In: The Cochrane Library, Issue 3, 2000. Oxford: Update Software.
93. Alrajhi AA. Urinary tract infection in spinal cord injury patients. Saudi Med J 1999;20:24-28.
94. Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: A prospective study of 1497 catheterized patients. Arch Intern Med 2000;160:678-682.
95. Abrutyn E, Mossey J, Berlin JA, et al. Does asymptomatic bacteriuria predict mortality and does antimicrobial treatment reduce mortality in elderly ambulatory women? Ann Intern Med 1994;120: 827-833.
96. Warren JW, Damron D, Tenney JH, et al. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis 1987;155:1151-1158.
97. Geerlings SE, Stolk RP, Camps MJ, et al. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care 2000;23:1737-1741.
98. O’Hanley P, Lark D, Falkow S, et al. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest 1985;75:347-360.
99. Sobel JD. Pathogenesis of urinary tract infection. Role of host defenses. Infect Dis Clin North Am 1997;11:531-549.
100. Svanborg Eden C, et al. Host-parasite interaction in the urinary tract. J Infect Dis 1988;157:421-426.
101. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993;329:1328-1334.
102. Ronald A, Nicolle LE, Harding G. Single dose treatment failure in women with acute cystitis. Infection 1992;20(Suppl 4):S276-S279.
103. Busch R, Huland H. Correlation of symptoms and results of direct bacterial localization in patients with urinary tract infections. J Urol 1984;132:282-285.
104. Jones SR, Smith JW, Sanford JP. Localization of urinary-tract infections by detection of antibody-coated bacteria in urine sediment. N Engl J Med 1974;290:591-593.
105. Sultana RV, Zalstein S, Cameron P, et al. Dipstick urinalysis and the accuracy of the clinical diagnosis of urinary tract infection. J Emerg Med 2001;20:13-19.
106. Jenkins RD, Fenn JP, Matsen JM. Review of urine microscopy for bacteriuria. JAMA 1986;255:3397-3403.
107. Bailey BL Jr. Urinalysis predictive of urine culture results. J Fam Pract 1995;40:45-50.
108. Komaroff AL. Urinalysis and urine culture in women with dysuria. Ann Intern Med 1986;104:212-218.
109. Morgan MG, McKenzie H. Controversies in the laboratory diagnosis of community-acquired urinary tract infection. Eur J Clin Microbiol Infect Dis 1993;12:491-504.
110. Lammers RL, Gibson S, Kovacs D, et al. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med 2001;38:505-512.
111. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med 1983;75(1B):53-58.
112. Blum RN, Wright RA. Detection of pyuria and bacteriuria in symptomatic ambulatory women. J Gen Intern Med 1992;7:140-144.
113. Kusumi RK, Grover PJ, Kunin CM. Rapid detection of pyuria by leukocyte esterase activity. JAMA 1981;245:1653-1655.
114. Gelbart SM, Chen WT, Reid R. Clinical trial of leukocyte test strips in routine use. Clin Chem 1983;29:997-999.
115. Winkens RA, Leffers P, Trienekens TA, et al. The validity of urine examination for urinary tract infections in daily practice. Fam Pract 1995;12:290-293.
116. Holloway J, Joshi N, O’Bryan T. Positive urine nitrite test: An accurate predictor of absence of pure enterococcal bacteriuria. South Med J 2000;93:681-682.
117. Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians 1956;69:56.
118. Kass EH. Bacteriuria and the diagnosis of infections of the urinary tract. Arch Intern Med 1957;100:709-714.
119. Kunin CM, White LV, Hua TH. A reassessment of the importance of "low-count" bacteriuria in young women with acute urinary symptoms. Ann Intern Med 1993;119:454-460.
120. Arav-Boger R, Leibovici L, Danon YL. Urinary tract infections with low and high colony counts in young women. Spontaneous remission and single-dose vs multiple-day treatment. Arch Intern Med 1994;154:300-304.
121. Stamm WE, Counts GW, Running KR, et al. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med 1982;307: 463-468.
122. Fairley KF, Carson NE, Gutch RC, et al. Site of infection in acute urinary-tract infection in general practice. Lancet 1971;7725: 615-618.
123. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med 1980;303:409-415.
124. Lipsky BA, Inui TS, Plorde JJ, et al. Is the clean-catch midstream void procedure necessary for obtaining urine culture specimens from men? Am J Med 1984;76:257-262.
125. Lipsky BA, Ireton RC, Fihn SD, et al. Diagnosis of bacteriuria in men: specimen collection and culture interpretation. J Infect Dis 1987;155:847-854.
126. Harwood-nuss A, Etheredge W, McKenna I. Urological Emergencies. In: Emergency Medicine Concepts and Clinical Practice. Barkin, Ed. St. Louis: Mosby; 19982227-2261.
127. Nicolle LE, Harding GK, Kennedy J, et al. Urine specimen collection with external devices for diagnosis of bacteriuria in elderly incontinent men. J Clin Microbiol 1988;26:1115-1119.
128. Kawashima A, Sandler CM, Goldman SM. Imaging in acute renal infection. BJU Int 2000;86(Suppl 1):70-79.
129. Hoddick W, Jeffrey RB, Goldberg HI, et al. CT and sonography of severe renal and perirenal infections. AJR Am J Roentgenol 1983; 140:517-520.
130. June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985;145:841-845.
131. Henry DC, Riffer E, Haverstock DC, et al. Once-daily extended release ciprofloxacin vs. conventional twice-daily ciprofloxacin for the treatment of uncomplicated urinary tract infections. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, Sept. 27-30, 2002; San Diego, CA. Abstract L-1800 (oral presentation, E. Riffer, Sept. 30, 2002).
