Drug Criteria & Outcomes - Alefacept (Amevive) Formulary Review
By Miranda B. Chambers, PharmD candidate
Harrison School of Pharmacy
Auburn (AL) University
Alefacept (Amevive), produced by Biogen, represents a new drug class for patients with chronic plaque psoriasis. The drug was approved by the U.S. Food and Drug Administration (FDA) on Jan. 31, 2003, and became available on Feb. 3, 2003.
Mechanism of action
Alefacept interferes with lymphocyte activation, via binding to CD2, the lymphocyte antigen, and inhibiting the interaction between CD2 and LFA-3, the leukocyte-associated function antigen, which promotes cell-cell interaction. LFA-3 on the antigen-presenting cell and CD2 on T-lymphocytes interact in the pathophysiology of chronic plaque psoriasis.
Secondly, alefacept causes a reduction in subsets of CD2+ T-lymphocytes by attaching to the Fc(RIII IgG, which can engulf multivalent complexes, causing apoptosis in those cells expressing high levels of CD2. Because CD2 is higher on memory cells than naïve cells, there is a selective reduction in circulating total CD4+ (helper cells) and CD8+ (suppessor or cytotoxic cells) T-lymphocyte counts. CD4+ helper cells stimulate other cells in the immune response. CD8+ suppressor cells help to down-regulate the immune response once the pathogen is destroyed. CD8+ cytotoxic cells kill cells that are recognized as foreign. However, this effect seems to be dose-dependent.
Pharmacokinetics
In clinical trials, efficacy was correlated with the serum levels of the drug. However, the package insert does not recommend routine monitoring of levels, and a therapeutic range for drug levels has not been established. Bioavailability is 63%.
Monitoring parameters
White blood cell (lymphocyte) count should be monitored weekly during treatment.
Indication
Alefacept is indicated for treatment of adults with moderate-to-severe chronic plaque psoriasis who are candidates for systemic therapy or photo-therapy.
Dosage
Intramuscular (IM) dosage is 15 mg once weekly for 12 weeks, and can be repeated after a 12-week treatment-free period if CD4+ count is normal.
As of Oct. 3, 2003, Biogen discontinued a 7.5 mg intravenous (IV) dosage form because more dermatologists preferred the IM injection.
Drug interactions
Formal studies are not available for drug interactions with alefacept. Due to the mechanism of action, alefacept may have additive effects when administered concurrently with other drugs that suppress T-cell activation, such as cyclosporine.
Adverse effects
The most serious adverse effects were:
- Lymphopenia (decreased CD4+ and CD8+ counts)
- Malignancies
- Serious infections requiring hospitalization (immunosuppressive properties)
- Hypersensitivity reactions (urticaria, angioedema, anaphylaxis)
The most commonly occurring adverse events, which required clinical intervention, were cardiovascular events: coronary artery disease (< 1%) and myocardial infarction (< 1%).
The most common adverse events causing discontinuation of the drug included: decreased CD4+ T-lymphocyte levels (< 250 cells/mcL), headache (0.2%), and nausea (0.2%).
Contraindications
The only contraindication to alefacept at this time is hypersensitivity to alefacept or any component of the drug.
Precautions
- Immunosuppressive effects occurred, so other immunosuppressive agents or phototherapy should not be coadministered.
- Hypersensitivity reactions occurred when alefacept was administered. If anaphylaxis occurs, alefacept should be discontinued.
- Decrease in T-lymphocytes occurred, so weekly monitoring of white blood cells should be done during the treatment periods. If the CD4+ level falls below 250 cells/mcL, the dose should be withheld. If the count stays below 250 cells/mcL for longer than one month, the drug should be discontinued.
- Malignancies may occur, but no definitive data exist.
- Effects on fetal development are unknown, but a large number of the patients with psoriasis are women of childbearing age. Therefore, women who are pregnant and decide to take alefacept are asked to enroll in a Biogen Pregnancy Registry [(866) AMEVIVE]. Studies performed in monkeys showed no abortifacient or teratogenic effects, so it is rated Pregnancy Category B.
- Excretion in breast milk is undetermined.
- Geriatric patients are more susceptible to some malignancies and infections, so caution should be used when treating this population. However, in a study of 1,357 patients, no differences were shown between different age groups using alefacept.
- Safety and efficacy have not been studied in the pediatric population.
To reduce error potential, physician clinics should be educated that alefacept should be administered IM, not IV.
Clinical studies
Study No. 1: Ellis C, Krueger G. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001;345:248-255.
Objective: To evaluate alefacept as a treatment for psoriasis.
Study design/patient population: Multicenter, randomized, placebo-controlled, double-blinded, parallel-group study involving 229 patients. Study included men and women, ages 18-70 with chronic plaque psoriasis involving at least 10% of body surface area for at least 12 months before study.
Inclusion criteria: Previous phototherapy
or systemic treatment or candidates for those treatments.
Exclusion criteria
- Serious hepatic or renal disease
- History of cancer (except basal cell carcinoma or less than three squamous cell carcinomas of the skin)
- Weight of 75% or more above ideal body weight
- Serious infection in previous three months
- Women of childbearing age, unless using contraception
- Systemic treatments, phototherapy, or potent topical treatments within previous four weeks (and not until two weeks after treatment)
- Moderate-potency topical corticosteroids, keratolytics, coal tar, or calcipotriene were restricted to use on groin, scalp, palms, and soles
Endpoints: The endpoints for the study included safety and efficacy.
Treatment regimens: Patients were randomly assigned to receive alefacept 0.025 mg/kg, 0.075 mg/kg, or 0.150 mg/kg, or placebo. Alefacept
was administered by 30-second IV injection once weekly for 12 weeks.
Results: During the 12-week treatment phase, patients receiving alefacept had significantly better improvement in psoriasis area and severity index (PASI) than those in the placebo group (P < 0.001).
- Two weeks after treatment, significantly more patients from the alefacept groups still had at least 50% reduction in PASI.
- Twelve weeks after treatment, still significantly more alefacept patients had greater than 50% reduction (P = 0.02). There were no reports of flare or rebound of disease after treatment ended.
This study showed a correlation between serum levels and drug efficacy. There was also a dose-dependent decrease in peripheral-blood CD4+ memory effector cells. Alefacept was well-tolerated. No adverse event was more than 5% greater in the alefacept group vs. placebo.
Conclusion: The authors concluded that once-weekly alefacept for 12 consecutive weeks was an effective and well-tolerated treatment for chronic plaque psoriasis.
Strengths
- Adequate sample size
- Study design
- P values and percentages stated
- Intention-to-treat method used
- Appropriate statistical tests used
Limitations
- Confidence interval and power were not stated.
- No patient specific data were given.
Study No. 2: Krueger GG, Papp KA, Stough DB, et al. A randomized, double-blind, placebo-controlled Phase III study evaluating efficacy and tolerability of two courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 2002;47:821-833.
Objective: To evaluate the efficacy and tolerability of two courses of alefacept in a phase III study of patients with chronic plaque psoriasis.
Study design/patient population: Rando-mized, double-blind, placebo-controlled, parallel-group study involving 553 patients (166 females and 387 males). Patients, ages 16-84, were randomized to three groups, either receiving two courses of alefacept or one course of alefacept and one course of placebo.
Inclusion criteria
- Chronic plaque psoriasis for at least 12 months prior to trial (10% or more of body surface area)
- CD4+ lymphocyte count greater than lower limit of normal
Exclusion criteria
- Systemic or serious local infection within three months prior to study
- History of malignancy
- Pregnant or nursing females
- Some treatments within four months of the study (phototherapy and some drug therapy)
- Some treatments within two weeks of and throughout the study, except on the scalp, palms, groin, soles (moderate-potency topical corticosteroids, vitamin D analogs, keratolytics, and coal tar)
- Therapeutically ineffective low-potency topical corticosteroids and emollients within 12 hours of the endpoint measurements
Endpoints: The primary endpoint was efficacy, which was defined as 75% or greater decrease in PASI at two weeks after course one was completed. The secondary endpoints were safety and therapy duration, especially looking at the second course of alefacept.
Treatment regimens: Most patients (548) received a 7.5 mg dose once weekly for 12 weeks, while five patients (weighing less than 50 kg) received 5 mg once weekly. Each dose was a 30-second IV injection.
Results: Four hundred eighty-two patients completed course one treatment and follow-up and began course two; 401 patients completed course two treatment and follow-up. Results of the study are detailed in Table 1.
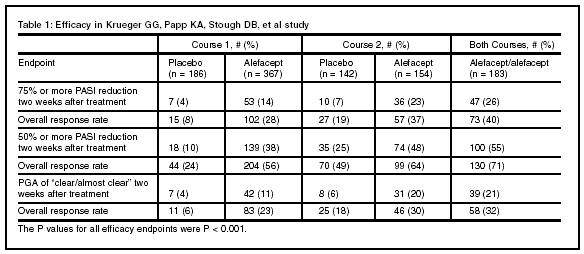
Duration of response
- Single course of alefacept (cohort 2): Those who achieved 75% or more reduction in PASI maintained 50% or more reduction in PASI for a median duration of more than seven months.
- Single course of alefacept (cohort 2): Those who achieved PGA of "clear/almost clear" during or after treatment maintained a 50% or more reduction in PASI for a median duration of about eight months.
- Double courses of alefacept (cohort 1): Those who achieved 75% or more reduction in PASI or physician global assessment (PGA) of "clear/almost clear" maintained a 50% or greater response longer than the final endpoint of the study (one year); no median duration could be determined.
The duration of 50% or greater reduction in PASI was significantly longer in cohort 1 vs. cohort 2 among patients who achieved a 75% or greater reduction in PASI (P = 0.019) or PGA of "clear" or "almost clear" (P = 0.35) at any time during the study. This predicts that two courses produce longer duration than one course.
Tolerability
- Alefacept was well-tolerated.
- Alefacept caused chills, elevation of ALT (cohort 2), and CD4 depletion more than placebo.
Conclusion: In this study, the authors concluded that either one or two courses of IV alefacept produced significant clinical improvements in measures of chronic plaque psoriasis. There was a further increase in efficacy with the second course of treatment. Alefacept was well-tolerated, and the duration of treatment was longer than currently available treatments. Because psoriasis is a chronic disease that fluctuates from remission to disease flare, it is frequently managed with significantly toxic drugs. Alefacept may help meet the need for safe and effective remittive therapy.
Strengths
- Study design
- Large sample size
- Intention-to-treat analysis used
- P values and percentages stated
- Appropriate statistical tests used
Limitations
- Confidence interval not stated
- No long-term data (of more than one year)
- More males than females
- Sample size needed (555) vs. actual (553)
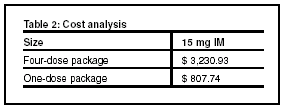
Cost
A cost analysis is provided in Table 2. Biogen offers an assistance program to uninsured patients, which is available through an application process.
Storage
Store the dose tray of lyophilized powder at controlled room temperature (15-30° C or 59-86° F). It should be protected from light and remain in the carton until used.
Conclusions and recommendation
Based on currently available literature, alefacept is a novel drug therapy for chronic plaque psoriasis. It is different from currently available treatments in that it targets specific lymphocytes involved in the immune response and has a longer duration of action. It has proven safe and effective in clinical trials, with two treatment courses being even more effective than one course.
Due to the dosage form and administration, it generally will be administered in physician offices or clinics (see Table 3 for a criteria for use tool). For this reason, the drug is recommended to be non-formulary status, and not stocked in the hospital. If an inpatient on alefacept therapy requires a dose while in the hospital, the prearranged home supply should be used. Initiation of therapy should be deferred to the outpatient setting.

Additional resources
- Biogen Amevive package insert. Cambridge, MA; 2003.
- Anonymous. Alefacept (Amevive) for treatment of psoriasis. Med Lett Drugs Ther 2003;45:31-32.
- Krueger GG, Callis KP. Development and use of alefacept to treat psoriasis. J Am Acad Dermatol 2003;49:87-97.
- Pham DQ, Bandy V, Song JC. Alefacept: A T-cell-specific immunosuppressant to treat moderate to severe plaque psoriasis. Formulary 2002;37:346-353.
Alefacept (Amevive), produced by Biogen, represents a new drug class for patients with chronic plaque psoriasis. The drug was approved by the U.S. Food and Drug Administration (FDA) on Jan. 31, 2003, and became available on Feb. 3, 2003.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
