Drug Criteria & Outcomes: Apomorphine Hydrochloride (Apokyn) Formulary Evaluation
Drug Criteria & Outcomes: Apomorphine Hydrochloride (Apokyn) Formulary Evaluation
By Jennifer Lightfoot,
PharmD candidate
McWhorter School of Pharmacy
Samford University, Birmingham, AL
Apomorphine was recognized in 1960 to affect dopamine receptors in the treatment of Parkinson’s disease (PD). The U.S. Food and Drug Administration (FDA) designated apomorphine as an orphan drug in 1991 for the treatment of hypomobility in idiopathic stage intravenous (IV) PD patients. At the conclusion of three clinical trials, the FDA approved apomorphine in April for the acute, intermittent treatment of unpredictable "on/off," hypomobility, or "end-of-dose wearing off" in advanced PD patients. Apomorphine hydrochloride is manufactured by Bertek Pharmaceuticals.
Mechanism of action
Apomorphine is a nonergoline
dopamine agonist. Apomorphine has an affinity for binding on the dopamine receptors,
especially the D4 receptor. The drug possesses a more moderate affinity
for the receptors at the D2, D3, and D5, and
adrenergic 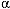 1D,
1D, 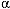 2B, and
2B, and 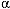 2C receptors. Apomorphine
has a low affinity for the dopamine D1, serotonin 5HT1A,
5HT2A, 5HT2B, and 5HT2C receptors. An affinity
for ß1 and ß2 or histamine H1 receptors also
is noted.
2C receptors. Apomorphine
has a low affinity for the dopamine D1, serotonin 5HT1A,
5HT2A, 5HT2B, and 5HT2C receptors. An affinity
for ß1 and ß2 or histamine H1 receptors also
is noted.
Apomorphine is classified as a dopamine agonist for PD; however, its exact mechanism of action in the treatment of PD is unknown. It is thought to stimulate post-synaptic D2 receptors in the caudate-putamen section of the brain.
Pharmacokinetics
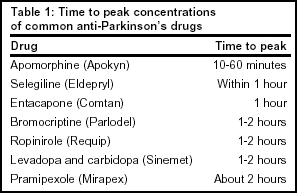
Absorption: Apomorphine is lipophilic and is rapidly absorbed. This plays an important role in its effectiveness to treat hypomobility. The time to peak concentration ranges from 10-60 minutes following a subcutaneous injection into the abdominal wall (Refer to Table 1, below, for comparison to other PD treatments.)
Distribution: The mean of apparent volume of distribution is 218 L. Maximum concentrations in the cerebrospinal fluid (CSF) are less than 10% of the maximum plasma concentrations and occur 10-20 minutes later.
Metabolism and elimination: The mean apparent clearance is 233 L/hr. The elimination half-life is about 40 minutes (range 30-60 minutes). The route of metabolism in humans is unknown.
Special populations: The clearance of apomorphine does not appear to be influenced by age, gender, weight, duration of PD, levadopa dose, or duration of therapy.
Hepatic impairment: The area under the curve (AUC)0-infiniti and maximum concentration (Cmax) values were increased by 10% and 25%, respectively, in hepatic patients when compared to healthy patients following a subcutaneous injection of apomorphine.
Renal impairment: The AUC0-infiniti and Cmax values were increased by 16% and 50%, respectively, in renal patients when compared to healthy patients following a subcutaneous injection of apomorphine.
Both hepatic and renal impairment would require a dose adjustment. Specific dosing guidelines for the use of apomorphine in patients with hepatic and renal failure currently do not exist.
Dosing and administration
The manufacturer recommends the use of trimethobenzamide (Tigan) 300 mg three times a day (tid) orally to be started three days prior to initial dose to decrease the incidence of nausea and vomiting. The 5HT3 antagonists should not be used because of potential interactions. The combination may lower blood pressure and cause patients to lose consciousness and blackout.
The dose of apomorphine should be based on effectiveness and toleration. Start the dose at 0.2 mL (2 mg) and titrate up to a maximum dose of 0.6 mL (6 mg) or a total daily dose of 20 mg. The average daily dose in most patients is 0.3 mL (3 mg) to 0.6 mL (6 mg) three times daily.
Start patients with a test dose of 0.2 mL (2 mg). Then begin an increasing titration of 0.1 mL (1 mg) increments every few days on an outpatient basis. If patients do not respond to the test dose, then increase at the next observed off time to 0.4 mL (4 mg). If the patient tolerates the 0.4 mL (4 mg) test dose, then therapy should be started at 0.3 mL (3 mg) on an as-needed basis. An increase in 0.1 mL increments can be made if needed.
When dosing patients with mild-to-moderate renal or hepatic failure, the initial test dose should be decreased to 0.1 mL (1 mg).
Administration: Apomorphine is administered as a subcutaneous injection. Patients should be instructed to change the injection site each time the drug is administered. Suggested sites are the abdomen, upper arm, and upper leg.
Pregnancy rating: Category C
Strengths and dosage forms: Apomorphine is supplied as solution (10 mg/1 mL) in 2 mL glass ampules (carton of five) and 3 mL cartridges (carton of five). The 3 mL cartridges are used with a manual reusable, multiple- dose injector pen. The pen can deliver doses up to 1.0 mL in 0.02 mL increments. Six needles and a carrying case are provided with the pen.
Adverse reactions, interactions, and contraindications
Apomorphine has a similar adverse drug reaction profile to that of other agents used in the treatment of PD, in that each has been associated with causing nausea, syncope, dyskinesia, somnolence, and hallucinations. The major side effect incidences for apomorphine hydrochloride vs. placebo are listed in Table 2, below.
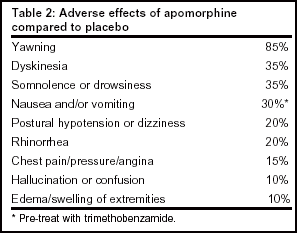
Drug-drug interactions: The drug should not be used in combination with 5HT3 antagonists due to the potential for hypotension and loss of consciousness. Apomorphine may cause hypotension if given in combination with antihypertensive medications and vasodilators, dopamine antagonists, and drugs that prolong the QT/QTc interval. Drugs that may aggravate PD symptoms should not be given concurrently with apomorphine (i.e., metoclopramide).
Contraindications: Apomorphine administered concomitantly with ondansetron may cause profound hypotension and loss of consciousness. The use of apomorphine with the 5HT3 antagonist class (including, for example, ondansetron, granisetron, dolasetron, palonosetron, and alosetron) is contraindicated.
Apomorphine is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients (notably sodium metabisulfite).
Warnings and precautions
AVOID INTRAVENOUS ADMINISTRATION: Serious adverse events (such as intravenous crystallization of apomorphine, leading to thrombus formation and pulmonary embolism) have followed the intravenous administration of apomorphine.
Dyskinesias: Apomorphine may cause dyskinesia or exacerbate pre-existing dyskinesia. During clinical development, dyskinesia, or worsening of dyskinesia was reported in 24% of patients.
Priapism: Apomorphine may cause prolonged painful erections in some patients.
Cost
As of June, only the average wholesale price of apomorphine was available per the manufacturer ($23/day + $3/day for trimethobenzamide).
Clinical trial summary
There are three clinical trials that support the use of apomorphine in Parkinson’s patients. All three trials used the Unified Parkinson’s Disease Rating Score (UPDRS) to measure clinical effectiveness. The UPDRS is a standard test that evaluates the patient’s motor skills, emotional and mental status, and overall physical status relating to the side effects of PD. The UPDRS primarily uses subjective data to evaluate a patient. The lower the overall score a patient receives the better the outcome. The scoring range is 0-176 points.
A prospective, randomized, parallel, double-blind, placebo-controlled study was conducted with 29 patients who had advanced idiopathic PD (Dewey et al, 2001). First, in an office setting, medications were withheld from patients overnight to direct a hypomobility state. Twenty patients were assigned to the treatment and nine to placebo. Patients given apomorphine were initiated at a 0.2 mL (2 mg) dose and then titrated by 0.2 mL (2 mg) until the goal response (a 90% reduction in the UPDRS) was achieved; 18 patients in the treatment group experienced a therapeutic response. The average dose of apomorphine hydrochloride was 5.4 mg. None of the patients receiving placebo experienced any benefit. The mean changes in the UPDRS Part III scores were a reduction of 23.9 points for apomorphine and 0.1 point reduction for the placebo group (P < 0.0001). The outpatient phase consisted of the patients continuing their regular anti-PD medications along with the highest titrated apomorphine dose received in the inpatient phase. The patients were evaluated for 30 days. After two weeks, the option of titration of the apomorphine dose was allowed. The results of the outpatient phase were a two-hour reduction in "off" hours per day by the apomorphine group and no hours reduced in the placebo group. Apomorphine aborted 95% of the "off" events, while placebo only aborted 23%.
Sherry et al conducted a prospective, randomized, placebo-controlled, crossover design to measure the safety and effectiveness of subcutaneous apomorphine in the treatment of "off" episodes in PD patients. The study sample consisted of 62 participants who had been using apomorphine for at least three months and were optimized on their PD regimen. Patients were placed into four groups. The first group received apomorphine at the regular dose plus 0.2 mL additional apomorphine. The second group received apomorphine at the regular dose plus 0.2 mL placebo. The third group received placebo. Finally, the fourth group received apomorphine at the regular dose.
Efficacy was determined by the change in the UPDRS. The doses were given at the first sign of an "off" episode. The results at 10 and 20 minutes for the post-dose changes were a reduction in the UPDRS of 19.9 and 24.4 points, respectively, for the pooled apomorphine groups. The placebo groups pooled results, after 10 and 20 minutes, posted a reduction of 5.6 and 7.4 points, respectively.
In a prospective, randomized, placebo-controlled, crossover study, Bertek Pharmaceuticals (unpublished data in product insert) examined the safety and effectiveness of subcutaneous apomorphine in the treatment of "off" episodes in PD patients. The study sample consisted of 17 participants who had been using apomorphine for at least three months in addition to oral PD medications. Patients received a single dose of apomorphine at their regular dose or placebo at the same volume amount. The dose was initiated at the first "off" event occurring after administration of the morning PD medications. The efficacy was measured as a change in the UPDRS. The results were a baseline change of 20 points for apomorphine and 3 points for placebo on the UPDRS.
The trials discussed here show the advantages of using apomorphine to treat the hypomobility of PD. More research needs to be conducted to compare apomorphine to other PD medications. The trials were similar in having a small sample size, which does not adequately represent the population. Likewise, the trials use the UPDRS to evaluate the efficacy of using apomorphine, which may introduce bias into the trial because the data collected are subjective data. Statistical analysis information was only available for the Dewey trial. The trial used intention-to-treat analysis with an expected 10%-20% dropout rate (three of the 29 participants dropped out) and a power of 87%, allowing a 13% chance of a Type 2 error.
Place in therapy/advantages
Apomorphine is considered a rescue drug for PD patients who repeatedly experience hypomobility periods due to the wearing-off of anti-PD medications (usually levadopa). The hypomobility stages can be disabling to the patient. Some patients rebut to an "off" state with panic attacks, screaming, or excessive sweating. A main benefit of use is reduced incidence and time of hypomobility ("off" periods). Compared to other anti-PD agents, apomorphine on average reduces the "off" period by two hours. The only other Parkinson’s therapies that are comparable are ropinirole and tolcapone, which reduced the off period on average by 1.9 and three hours, respectively, in noncomparative trials.
The major advantage of apomorphine is that it demonstrates the fastest onset of action of all the PD drugs, acting in as fast as 10 minutes; no other anti-Parkinson’s agent can compare with this rapid onset of action. The onset of action also is convenient for patients who suspect that an "off" state is approaching and can quickly combat the state. Apomorphine’s positive clinical effects can last up to an hour.
Formulary considerations
It is recommended that apomorphine be classified as formulary status. It should be limited formulary status for advanced PD patients where other drugs have not adequately controlled acute "on-off" and "end-of-dose" hypomobility episodes. A small drug supply will be maintained. Compared to the other anti-PD medications, the main advantage of apomorphine is more rapid onset.
Steps should be taken to manage the possibilities of medication errors and other safety issues: "look-alike, sound-alike" confusion with morphine, subcutaneous administration only, need for concurrent anti-nausea therapy, contraindication with 5-HT3 antagonists or metoclopramide, QTC interval prolongation, dosage adjustment in renal/hepatic dysfunction, and possible worsening of dyskinesias.
Resources
- Belden H. New rescue drug cleared for Parkinson’s "off-periods." Drug Topics. May 17, 2004;148:17.
- Bertek. Apokyn [package insert]. Research Triangle Park, NC; 2004.
- Bowron A. Practical considerations in the use of apomorphine injectable. Neurology 2004;62(Suppl 4):S32-S36.
- Dewey RB. Management of motor complications in Parkinson’s disease. Neurology 2004;62(Suppl 4):S3-S7.
- Dewey RB, Hutton T, LeWitt PA, et al. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for Parkinsonian off-state events. Arch Neurol 2001;58:1,385-1,392.
- Dipiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New York City: McGraw-Hill; 2002:1,089-1,099.
- U.S. Food and Drug Administration. FDA approves Apokyn for the acute treatment of episodes of immobility in Parkinson’s patients. Available at: www.fda.gov/bbs/topics/ANSWERS/2004/ANS01284.html. Accessed June 3, 2004.
- Lees A. Drugs for Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2002;73:607-610.
- Lexi-Comp (Lexi-Drugs. comp+ specialties) [computer program]. Lexi-Comp; June 5, 2004.
- Ostergaard L, Werdelin L, Odin P, et al. Pen-injected apomorphine against off phenomena in late Parkinson’s disease: A double-blinded, placebo-controlled study. J Neurol Neurosurg Psychiatry 1995;58:681-687.
- Sherry JH, Guyton PJ, Van Lunen B, et al. Continued efficacy and safety of subcutaneous injections of Apokyn in treatment of "off" episodes in patients with Parkinson’s disease. Neurology 2003;60:A81.
- Stocchi F, Vacca L, DePandis MF, et al. Subcutaneous continuous apomorphine infusion in fluctuating patients with Parkinson’s disease: Long-term results. Neurol Sci 2001;22:93-94.
- Van Laar T, Jansen E, Essink A, et al. A double-blind study of the efficacy of apomorphine and its assessment in "off-periods" in Parkinson’s disease. Clin Neurol Neurosurg 1993;95:231-235.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
