Aprepitant (Emend) Formulary Evaluation
Aprepitant (Emend) Formulary Evaluation
Mechanism of Action, Indications, Special Populations, Adverse Reactions, Drug Interactions, and Precautions
By Omar Dudar, PharmD
candidate
Harrison School of Pharmacy
Auburn (AL) University
Written while on clinical
rotation at Huntsville (AL) Hospital
Mechanism of action
Aprepitant (Emend) is unique from other drugs used for chemotherapy-induced nausea and vomiting (CINV) in that it is a selective antagonist of substance P/neurokinin 1 (NK1) receptors. Aprepitant has negligible affinity for other anti-emetic directed receptors such as serotonin (5-HT3), dopamine, and corticosteroid targets. Aprepitant is capable of penetrating the central nervous system (CNS) and passing the blood-brain barrier to bind with NK1 receptors.
Blockade of the 5-HT3 receptors by ondansetron and granisetron antagonize effects of enterochromaffin cell release of serotonin resulting from chemotherapy treatments. Interruption of the stimulus of neurotransmitters to the chemotrigger zone in the fourth ventricle of the brain will help to block the resultant triggering of the salivation center, and respiratory, pharyngeal, gastrointestinal (GI), and abdominal muscle contraction leading to vomiting.
Together with dexamethasone and ondansetron, aprepitant has been shown in studies to act synergistically to inhibit both the acute and delayed phases of highly emetogenic chemotherapy such as cisplatin-related emesis.
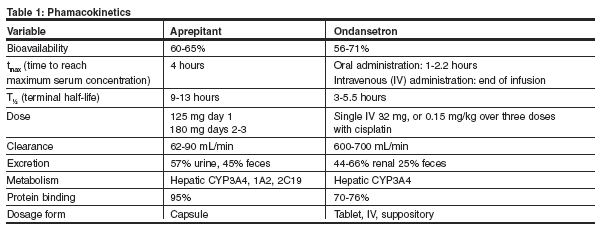
Pharmacokinetic profiles of aprepitant and ondansetron are summarized in Table 1.
Indication and dosage
Aprepitant is used in combination with dexamethasone and granisetron for acute and delayed nausea and vomiting due to highly emetogenic agents such as cisplatin and cyclophosphamide. Anticipatory emesis starts before administration of chemotherapy; acute emesis occurs within a few hours after starting chemotherapy, while delayed emesis starts after a day after chemotherapy. Aprepitant is given as a three-day regimen along with a corticosteroid and a 5-HT3 antagonist. The recommended dose of aprepitant is 125 mg one hour before beginning chemotherapy on the first day, and is combined with oral dexamethasone 12 mg and ondansetron 32 mg IV.
Dexamethasone 8 mg is given with each dose of 80 mg aprepitant for days 2 and 3. Ondansetron also is used separately or in combination with a corticosteroid for postoperative and radiation-induced nausea and vomiting. It is given as a single 32 mg IV dose or three 0.15 mg/kg doses intravenously for CINV. It is also available in a 4 mg dose to be administered over 2-5 minutes for postoperative prevention of nausea and vomiting.
Special populations (aprepitant)
Gender:
• Aprepitant has a 16% higher Cmax (maximum serum concentration) in females compared to males.
• Half-life is 25% lower in females.
• No dosage adjustment is necessary for differences.
Geriatric:
• Area under the curve (AUC)0-24h was 21% higher on day 1 and 36% higher on day 5 for elderly patients 65 years or older.
• No dosing adjustment is necessary.
Race:
• AUC0-24h is 25% and 29% higher in Hispanics vs. whites and blacks, respectively.
• Cmax is 22% and 31% higher in Hispanics compared to whites and blacks, respectively.
• No dosing adjustment is necessary.
Hepatic and renal insufficiency (aprepitant):
• No dosing adjustment due to clinically insignificant increases in AUC0-24h for mild-to-moderate hepatic and renal insufficiency.
• No dosage adjustment is necessary for aprepitant in severe renal insufficiency (creatinine clearance [CrCl] < 30 mL/min) and patients with end-stage renal disease (ESRD) requiring dialysis.
•• In patients with severe renal insufficiency and ESRD on dialysis, the Cmax decreased by 32% compared to healthy subjects.
- In severe renal insufficiency, AUC decreased by 21%.
- In ESRD patients undergoing dialysis, AUC decreased by 42%.
• • Although the AUC of total aprepitant (bound and unbound to protein) and Cmax decreased in both cases above, subsequent decreases in protein binding of aprepitant restore pharmacologically active unbound drug levels.
• • Formulary packet information indicates the resultant AUC of pharmacologically active unbound drug was not significantly affected in patients with renal insufficiency compared to healthy patients.
• There are no studies in severe hepatic insufficiency for recommendations of dosing changes.
Ondansetron: Do not exceed a total daily dose of 8 mg in severe hepatic dysfunction.
Adverse reactions
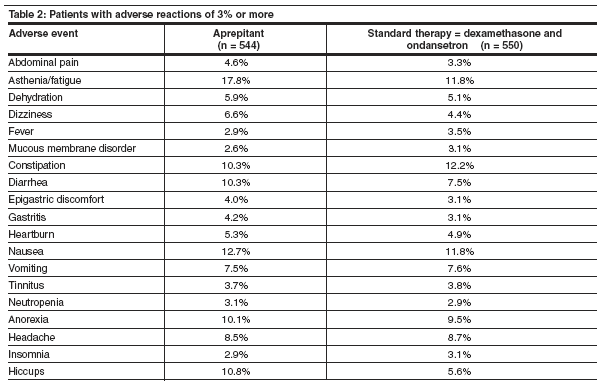
Adverse events were reported in 69% of 544 patients treated with aprepitant vs. 68% of 413 patients in the standard group. The most common adverse events of the aprepitant group were asthenia, headache, dizziness, constipation, diarrhea, abdominal pain, and anorexia (see Table 2). Hiccups and fatigue were more common with aprepitant-tested patients than standard therapy.
Drug interactions/precautions
Aprepitant is a substrate, a moderate inhibitor, and an inducer of CYP3A4 enzyme. Aprepitant also has demonstrated induction of CYP2C9 enzyme. Although aprepitant’s inhibition of CYP3A4 enzymes causes increases of other drugs metabolized by the CYP3A4 enzyme system, dosage adjustments of aprepitant were not made in trials.
Aprepitant’s concentration will greatly increase when given in addition to CYP3A4 inhibitors (ketoconazole, itraconazole, clarithromycin, and ritonavir). It is important to avoid administering aprepitant with known inducers of CYP3A4 (rifampin, carbamazepine, and phenytoin.) As an example of aprepitant’s induction of CYP2C9, levels of drugs such as the S-warfarin isomer and phenytoin may be lowered.
Ondansetron is a substrate for CYP1A2, 2D6, and 3A4, so inducers (rifampin, carbamazepine, clarithromycin) and inhibitors (cimetidine) of these enzymes will affect the levels of ondansetron. Ondansetron will decrease the analgesic effect of tramadol when used in combination. Ondansetron will decrease levels of cyclophosphamide when co-administered.
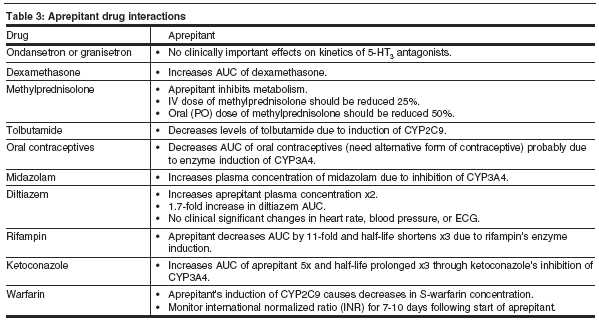
A list of drugs that interact with aprepitant therapy and the clinical effects of the interaction can be found in Table 3.

Ondansetron interactions QTc interval: Adenosine, antipsychotics (drug class), astemizole, bepridil, cisapride, clarithromycin, anti-arrhythmic agents (Class I and III), clindamycin, erythromycin, and fluconazole.
Summary: QTc interval prolongation is not a class-wide effect with the notable exception of granisetron. Dolasetron, however, produces prolongation of the QTc interval to the same extent as ondansetron.
Contraindications: Do not use aprepitant concurrently with CYP3A4 metabolism-dependent drugs such as pimozide, terfenadine, astemizole, or cisapride due to the increased possibility of QTc interval prolongation and risk of cardiac arrhythmias.
Ondansetron: Contraindicated if there is a hypersensitivity to 5-RAs.
Pregnancy/lactation
Aprepitant and ondansetron:
• Category B.
• No impaired fertility or harm to fetus known at this time.
• Excreted in the milk of rats.
•• No tests in humans.
•• Recommendation to stop breast-feeding or stop drug.
Possible medication errors with aprepitant
• Giving aprepitant at two different doses (125 mg on day 1 and 80 mg days 2 and 3).
• Specifying dosing times: Administer 125 mg aprepitant one hour before chemotherapy on day 1, and then patients receive 80 mg aprepitant once a day on days 2 and 3.
• Treating with aprepitant for more than three days.
• Using aprepitant with standard therapy for less highly emetogenic drugs.
• Giving aprepitant to stop an acute onset of vomiting.
Mechanism of Action, Indications, Special Populations, Adverse Reactions, Drug Interactions, and PrecautionsSubscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
