Systematic approach taken in evaluating sepsis drug
Systematic approach taken in evaluating sepsis drug
Focus on disease state, not drug management
After the results of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial were published in 2001, many institutions established criteria for use of drotrecogin alfa (activated) that were similar to the inclusion criteria used in the trial. Institutions, however, then had to decide how to evaluate the criteria and the outcomes of patients with sepsis. At one institution, a broad, systematic approach to that evaluation was taken.
Hospitals especially want to make sure that they are using drotrecogin alfa on the patients who will benefit most from it, says Shane Winstead, PharmD, intensive care unit (ICU) clinical pharmacy specialist in the Department of Pharmacy Services, and assistant professor at the College of Pharmacy, University of Kentucky Chandler Medical Center in Lexington. First, the medical center set up the criteria used in PROWESS for drotrecogin alfa (activated). One change was made, however, based on the approval of the expensive drug from the U.S. Food and Drug Administration (FDA).
"PROWESS included all patients who presented with specific criteria for severe sepsis with at least one organ failure," she says. "But when the FDA looked at the product, they only approved it for patients with high-risk of mortality." Therefore, Winstead and her colleagues decided to use the drug on the sicker patients, such as those with more organ dysfunction.
When criteria like these are set up, it is key to follow up later to see if they are being followed, Winstead says. "It’s for the cost and the safety of the drug — to make sure that we are selecting patients who aren’t high risk for bleeding and that we are minimizing our adverse events."
Winstead collaborated with Craig Martin, PharmD, antimicrobial management team pharmacist at the University of Kentucky and Jeff Durthaler, MS, RPh, outcomes liaison for Eli Lilly & Co. in Indianapolis. Their systematic approach began by looking at "safe, rational, and cost-effective" in terms of the drug and then defining one outcome of value that would be the best predictor of those three endpoints, she explains. "For each outcome, we decided on outcome influencers, those things that influenced whether the outcome would be positive or negative."
They chose bleeding, mortality, and total cost per life saved as the outcome of value for the safe, rational, and cost-effective endpoints. Mortality was important because many institutions are finding their rates to be higher than what was reported in PROWESS, Winstead says. "We were trying to look at Is that OK?’ Our target may not be the PROWESS outcome, but how close do we accept it around that outcome?"
Their evaluation found overall positive results but also specific areas that needed improvement. In terms of safety, they were happy with the outcomes and the patients’ adverse events, she notes. "We haven’t had any severe bleeding in any of our patients." A chart review, however, found more cases of moderate bleeding than they had expected.
"It is really not that clinically significant. The patients have some oozing around catheter sites or bruising; they don’t require transfusions," she says. A closer look found that staff weren’t following the package insert 100% of the time for those kinds of procedures. "We saw areas of improvement for our institution. Others may find that they are treating patients at a higher risk of bleeding such as coagulopathies."
Winstead and her colleagues did find their mortality rate was still within what they determined to be an acceptable range for the type of patients that they had. "When we broke that down to the influencers of mortality, we were looking at organ dysfunction, time to treat, APACHE II scores, and patients who have co-morbidities. We were happy to see that we were treating patients early after onset of initial organ dysfunction, within 16 hours."
Institutions need to pay attention to the time to treat, she adds. "Are they waiting too long to start the drug? Or are they catching patients further along in their illness to where the institutions may have had better outcomes if they had been more aggressive early on?" Winstead says.
Winstead and her colleagues found that their higher mortality rate compared to PROWESS may be a reflection of treating a larger number of patients with comorbidities. "It’s a difficult call from patient to patient about how aggressive you are going to be. We did identify a few patients who were probably far enough along in chronic illnesses that treating the acute illness really didn’t change that outcome."
In the cost-effective area, they looked at factors such as length of stay in the ICU and in the hospital. Winstead says they are trying to make improvements by implementing a more standardized approach to management in these patients, supportively and outside the use of the drug. "The big way to bring the cost down in these patients is to try to shorten the patients’ ICU and hospital stay."
Overall, Winstead believes that the drug is giving them some advantage, and she is satisfied with the approach they took to evaluating its use. "I liked how we determined the one outcome that we thought was most indicative of how we wanted to look at the drug and how we identified some influencers for each outcome." The approach is systematic in that they could take their results, find out what drove those results, and then use that information to target areas for improvement (see Table below).
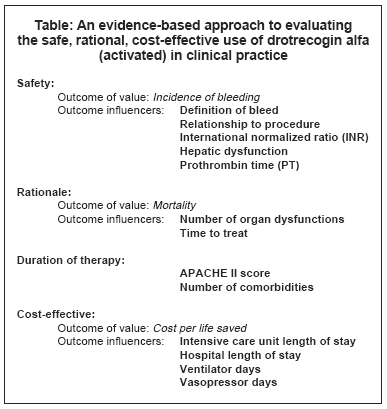
"We didn’t just focus on the drug itself," she says. "We tried to pull back and look at the disease state and how we managed that as a whole and how that impacted outcomes in our patients."
After the results of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial were published in 2001, many institutions established criteria for use of drotrecogin alfa (activated) that were similar to the inclusion criteria used in the trial. Institutions, however, then had to decide how to evaluate the criteria and the outcomes of patients with sepsis. At one institution, a broad, systematic approach to that evaluation was taken.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
