Management of the Difficult Airway
Authors: Thomas Nowicki, MD, FACEP, Integrated Residency in Emergency Medicine, University of Connecticut School of Medicine, Hartford Hospital, Hartford, Ct.; Shawn London, MD, Integrated Residency in Emergency Medicine, University of Connecticut School of Medicine, Hartford Hospital, Hartford, CT.
Peer Reviewers: Larry B. Mellick, MD, MS, FAAP, FACEP, Medical Consultant, Federal Bureau of Investigation Academy, Quantico, VA. Professor of Emergency Medicine and Pediatrics, Medical College of Georgia, Augusta; O. John Ma, MD, Professor and Vice Chair of Emergency Medicine, University of Missouri-Kansas City School of Medicine, Truman Medical Center.
Introduction
Basic airway management skills are vital to any practicing emergency medicine physician. Diagnosing, troubleshooting, and optimally managing the difficult airway often leads to a stressful and challenging situation. Education, practice, and experience provide improved physician comfort and better patient care.
Difficult airway is a broad term that unfortunately encompasses many clinical situations and their potential management options. Airway management difficulties can be divided into those that are predicted and those that are unexpected. They also can require immediate attention (emergent) or be stable (non-emergent). Difficulty can arise at any stage of airway management. For example, a patient can be difficult to mask ventilate while easy to intubate, as can be seen with significant mandibular trauma. Precise description of the difficulties encountered leads to better understanding and discussion of this topic.
Development of a standardized airway assessment and approach to management allows the physician to provide better patient care. Optimal care requires skill in assessing the situation, knowledge of equipment, and finally, how to use it successfully.
This article will attempt to focus on the more advanced conceptual aspects of this expansive topic and will not address the basic principles in the interest of brevity and clarity. We encourage our readers to seek additional resources such as Clinical Procedures in Emergency Medicine by Roberts and Hedges for specific procedural knowledge (e.g., cricothyroidotomy) where topics can be dealt with individually and at greater length.
Assessment of the Airway
Emergency physicians are responsible for some of the most challenging airway management cases in all of medicine. The work environment often provides chaotic circumstances with undifferentiated and critically ill patients who require respiratory assistance on an emergent basis. The American Society for Anesthesiologists’ Practice Guidelines for Management of the Difficult Airway (available at www.anesthesiology.org) is valuable for encouraging a systematic approach to the complex airway. However, as emergency physicians, our airway management algorithm cannot include direction to awaken the patient and reschedule his illness. What we can gain from this guideline is a structured approach to what is often a thorny situation.
In most cases, performance of an airway assessment should be done prior to managing the patient’s airway. Clearly, there are instances when the need to provide acute airway management precedes the ability to perform a complete history and physical examination. Even in these emergent circumstances, there usually is enough time to gather some information that can affect the provider’s decision-making and management plan. Airway assessment not only includes physical examination of the patient, but also consideration of the clinical scenario, available equipment/personnel, and the skill set of the airway physician.
The prevalence of patients who cannot be intubated within three attempts at direct laryngoscopy ranges between less than 1% to more than 13%, with most series falling between 3% and 5%.116 At least a brief evaluation of the patient’s airway prior to plunging headlong into standard rapid sequence and direct laryngoscopy is in the interest of avoiding an unexpected cannot-intubate-cannot-ventilate scenario. Numerous studies have proven that no single clinical measure will reliably identify those patients who will be problematic.2 However, in the authors’ clinical experience, we attempt to risk stratify patients as routine or possibly difficult by considering the aggregate appearance of several troublesome features reported in the literature. The possibly difficult patients clearly fall into a spectrum themselves. These predictors include short thyromental distance (i.e., length of lower jaw), decreased range of motion and length of the neck, high body mass index, dental factors (e.g., large frontal incisors, narrow occlusional distance or poor/missing teeth), facial structures (e.g., pronounced overbite or underbite), presence of a beard, and signs of obstructive sleep apnea. (See Table 1.) Reliance on the patient’s Mallampati classification is not always an ideal predictor of ease of intubation. In one retrospective series by Levitan and colleagues, less than one-third of the presenting patients were alert and compliant enough to complete an evaluation of these criteria.3 Facial hair, particularly a full beard, or structural abnormalities of the mandible and face should serve as a warning sign that the patient may be difficult to ventilate using a bag valve mask (BVM) if the initial intubation attempt is unsuccessful. Additionally, in the anesthesia literature, obstetric patients have been found to have more than three times the incidence of difficult airways compared with the average surgical patient (7.9% vs. 2.5 %, respectively).4
Awareness of these factors should lead the practitioner to gather additional personnel who can assist in ventilating the patient as well as retrieving the difficult airway cart or equipment. If difficulty is suspected, it is wise to seek additional skilled help before any attempts at intervention are made rather than waiting until the scenario worsens. Some patients who appear to be difficult on assessment can become much easier to manage than expected. The unfortunate corollary is that some of the many difficult intubations have no easily recognized predictor on assessment. These patients can pose a greater risk if the practitioner is caught unexpectedly without the necessary equipment or assistance needed to manage the situation.
In cases where management of the patient’s airway is predicted to be difficult, serious consideration should be given to an awake intubation. This technique can be performed with mild sedation (0.01-0.02 mg/kg midazolam) and topical analgesia of the oropharynx without paralysis (discussed further below). However, when dealing with combative patients or those with trismus, intubation can be performed much more easily with paralysis than with prolonged attempts with sedation alone. Particular care should be exercised in these situations; it can be quite difficult to accurately judge the ease of intubation prior to the administration of paralytic agents.
Positioning
There are many elements of patient position that can affect the difficulty of intubation. It cannot be stressed enough that optimal positioning should be sought prior to the first attempt at intubation regardless of the perceived difficulty. One should avoid having to make a second attempt at intubation solely because of poor positioning. Unnecessary additional attempts at intubation correspond with an elevated risk of complications (e.g., hypotension, bradycardia, aspiration and hypoxia).5-7
Identifying a patient who falls into the possibly difficult category should lead the provider to ensure that all of his or her available difficult airway equipment is close at hand prior to intubation efforts. A majority of patients can be positioned optimally with towels or blankets to approximate the desired sniffing position and align the airway axes. Some patients will be easier to intubate in alternative positions rather than the classic sniffing posture because of anatomic variations. The location of the patient on the stretcher and the stretcher height should be adjusted prior to providing care. Optimal positioning will not be possible in certain cases (e.g., those requiring cervical spine immobilization or for those patients who cannot tolerate a supine position). These challenges should serve as a sign of potential difficulty.
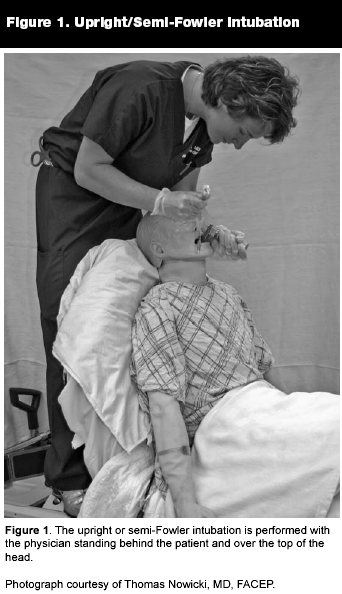
In the severely dyspneic patient (e.g., those with chronic obstructive pulmonary disease or congestive heart failure) who resists supine positioning, the operator may attempt an upright intubation in the Fowler's or semi-Fowler's position. (See Figure 1.) This position can be beneficial when electing to perform an awake intubation. A patient with obstructive sleep apnea, tongue swelling, or any extrinsic compression of the pharynx (e.g., hematoma, tumor) is at high risk for spontaneously obstructing the airway when his pharyngeal muscles relax and the architecture of his pharynx changes markedly from the awake state. Therefore, this group of patients is at high risk for difficulty with ventilation after being sedated and paralyzed. The upright/ awake technique preserves the tone of the musculature supporting the airway’s patency and maintains spontaneous respiration while allowing the physician to visualize the deeper laryngeal structures. If the vocal cords can be visualized, the operator, then, can time the passage of an endotracheal tube as the cords open and close during respiration. Easy visualization of the glottis in this position can serve as an indicator that the patient will be easy to intubate if paralysis becomes necessary. However, due to the previously mentioned changes in airway architecture, the operator occasionally may be surprised by a marked change in the laryngoscopic view when paralysis and sedation are added to the mix.
Building a ramp to position the obese and noncervical spine immobilized patient prior to intubation can lead to a major improvement in the laryngoscopic view. (See Figure 2.) This position will allow the airway axes to fall into better alignment by moving the pharyngeal and oral axes anterior to that of the tracheal axis. In these patients, the soft tissue mass overlying their back and shoulders can leave the position of the head quite far posterior, nearly opposite of the desired sniffing position. A ramp can be built with blankets starting at the waist and gradually inclining up the back and raising the head into a more typical sniffing position. The optimal view usually is obtained utilizing this technique when the tragus falls in line with the chest wall. This ramped position also helps diminish the effects of gravity as described in the upright intubation section above. The ramp technique should be considered prior to the first attempt at intubation for the significantly obese patient whose airway management plan includes direct laryngoscopy.
Primary Approach
Setup and planning are key elements that lead to success when performing any procedure. Emergency physicians currently have access to a large array of specialized airway management devices. With all of these options at hand comes the temptation to acquire and use as many of these tools as possible. It is important to develop a thorough understanding and expertise with a select number of these items to prevent misuse. Knowing when not to use a piece of equipment can be as valuable as knowing how to use the device properly.
The approach to any airway management situation should involve a well conceived primary approach and a detailed backup plan. Disaster can occur when the primary approach fails and the backup plan has not been prepared adequately. The authors would like to stress the importance of thinking through each step of the backup plan and gathering the items necessary to transform a theory into a viable option. Numerous variables can affect the plan chosen prior to the initiation of care.
Careful consideration of the clinical scenario and a thorough airway assessment usually will lead to several options for the initial airway approach. The particular provider’s skill set, equipment availability, and level of assistance available, then, will narrow the choices. Time urgency (emergent vs. non emergent) should be determined by the patient’s status and ease of ventilation. Direct laryngoscopy typically is the preferred primary approach particularly when no difficulty is anticipated. Direct laryngoscopy can be performed in conjunction with rapid sequence intubation as an awake intubation using only mild sedation or without any medication as in the post-arrest situation. Additional management options should be considered as part of the primary approach including nasotracheal, fiberoptic, laryngeal mask airway, retrograde wire, or even surgical airway depending upon the circumstances. The primary approach should be selected to provide the maximal chance for success with minimal risk to the patient. Nearly any airway device can be considered when developing the primary plan.
Rapid Sequence Intubation
Emergency physicians often are placed in situations where they must provide acute airway management without the luxury of a fully prepared patient. It is important to consider the timing of the patient’s last meal and the subsequent risk of aspiration during airway management. Rapid sequence intubation (RSI) was developed to minimize the risk of aspiration to the patient, while simultaneously creating a favorable setting in which to perform laryngoscopy. To competently perform this procedure, the emergency physician must develop and maintain a specialized knowledge base and skill set. Clearly, risks are associated with paralysis, and these must be assessed and weighed to preserve patient safety. Initial airway assessment and evaluation of the clinical scenario should help decide if RSI will be both safe to perform and beneficial. RSI should be used to transform a difficult airway into an easy airway to manage. However, potential exists to convert the spontaneously breathing patient with a self-protected airway into one who is apneic, difficult to ventilate, and at risk for aspiration.
The use of RSI has become standard among emergency physicians and when properly performed has been shown to be safe.8,9 Physicians who forget that this is a complex procedure fraught with potential for complications do so at their own peril. RSI can be defined as the administration of a potent sedative and a fast-acting neuromuscular blocking agent in rapid succession to facilitate endotracheal intubation while minimizing the risk of aspiration. This combination of drugs is intended to ablate both the stimulus for vomiting (e.g., anxiety, gag reflex) and the mechanism by which it occurs (i.e., ability of gastric musculature to contract and expel the stomach contents). Cricoid pressure (e.g., Sellick’s maneuver) must be applied concomitantly to prevent passive regurgitation. The basic steps to perform this procedure are not difficult, but they can be placed out of order or forgotten altogether, especially in a stressful situation. Close attention must be paid to the timing of these steps for RSI to be effective.
The steps of RSI often are remembered with the six Ps: prepare, preoxygenate, premedicate, paralyze, pressure on the cricoid cartilage, passage of the tube. Preparation for RSI should include organization of the proper equipment and the testing of its function. Failing to ensure that the proper equipment, personnel, and medications are ready prior to the start of RSI can distract from other important steps (e.g., properly positioning and oxygenating the patient). Poor positioning of the patient’s airway axes and stretcher height can lead to an unnecessary failed attempt. Every attempt should be made to maximize the likelihood of successful intubation on the first attempt.
Pre-oxygenation should be started as early as possible during the preparation phase of RSI. The goal in this step is to achieve nitrogen washout of the lungs. By replacing the ambient air inside the alveoli with near 100% oxygen, the time to oxygen desaturation can be increased greatly, allowing the physician more time to intubate. This high oxygen content in the alveoli creates a favorable oxygen gradient for the pulmonary circulation even in the absence of ventilation. Achieving nitrogen washout will require at least 2-3 minutes of normal respiration with a non-rebreather face-mask, 3-5 deep breaths by a conscious patient, or 5-10 full breaths via BVM. It is important to remember that the use of BVM-assisted ventilation during pre-oxygenation can elevate gastric pressure and, therefore, lead to increased risk of aspiration during intubation. Therefore, this practice should be avoided during a properly performed and routine RSI. Patients who are unable to elevate their oxygen saturation level adequately during pre-oxygenation (e.g., those with severe pulmonary edema) may require assisted ventilation prior to laryngoscopy. The benefit of improving the oxygenation level in these cases may outweigh the increased risk of gastric distension and aspiration. Gentle ventilation and use of cricoid pressure can help minimize gastric insufflation.
Appropriate medications should be actively chosen, drawn, and ready for administration prior to the start of this procedure. Detailed understanding of the pharmacologic profile of each medication used in RSI is vital. Knowledge of the onset and duration of action, side effects, and contraindications will help in choosing the best agents for a given clinical scenario. Selected patients can benefit from the administration of adjunctive medications prior to paralysis during RSI, commonly referred to as pre-medication. Pre-medication should be used to favorably augment the patient’s response to RSI. Laryngoscopy—as well as the agents used during RSI—can have side effects (e.g., alteration of heart rate, blood pressure and intracranial pressure). Lidocaine, atropine, narcotics, and defasiculating agents frequently are used adjuncts that fall into this group.
Lidocaine can be administered several minutes prior to paralysis in an attempt to minimize the rise in intracranial pressure during laryngoscopy. Its mechanism of action, however, is not known, and its efficacy is not proven clearly. There is some evidence that it is beneficial, and it does appear to be safe for use in those patients with known or suspected elevation of intracranial pressure.10 Atropine should be administered to children and to bradycardic patients prior to the use of succinylcholine and laryngoscopy due to their ability to increase vagal tone. Narcotic agents (e.g., fentanyl) also can help blunt the rise in intracranial pressure during laryngoscopy. A small defasiculating dose of a nondepolarizing paralytic agent (i.e., 0.01 mg/kg IV vecuronium) administered prior to the full dose of succinylcholine can be used to prevent skeletal muscle fasciculation during RSI. This technique can be used to prevent further injury in patients with a long-bone fracture to minimize rise in intraocular pressure (e.g., in the case of potential globe rupture), or to minimize any rise in intracranial pressure, for example. To obtain the proper effect, a defasiculating agent must be given several minutes before the paralyzing dose of succinylcholine. In rare cases, the administration of a defasiculating agent can decrease diaphragm and intercostal muscle strength leading to inadequate respiratory effort or even premature loss of spontaneous respiration. Therefore, it is mandatory that all equipment be set up and immediately ready for use and that the provider be prepared to intubate prior to the administration of any paralytic agent, regardless of the dose.
There are several classes of sedative agents available for use in RSI. The choice can be based upon their pharmacokinetic properties, as well as their side effect profile and their availability. Etomidate, propofol, and midazolam are some commonly used sedatives. The ideal sedative agent would have a rapid onset, short duration, be reversible, and possess amnestic properties. It also would have no effect on systemic vascular resistance, heart rate, or intracranial pressure. Etomidate is a carboxylated imidazole that closely fits this profile, hence its popularity in RSI. Myoclonus can occur after the administration of etomidate, but the paralytic agents used during RSI usually mask this effect. Adrenal suppression is another theoretical risk with this agent that has not been proven to be clinically significant, particularly when given as a single dose during RSI.11 This should be considered, however, in patients with known adrenal crisis or when administering repeat doses. Propofol is another commonly used sedative that has rapid onset and a brief duration of action; the agent rapidly redistributes out of the central nervous system and into adipose tissue. The dose-dependent decrease in blood pressure produced resulting from diminished arterial tone is particularly significant in patients who are borderline or frankly hypotensive prior to induction with this agent. Before the introduction of etomidate, midazolam was one of the most popular sedatives used during RSI because of its rapid onset and amnestic properties coupled with its reversibility and low cost. It can lead to hypotension and apnea, although these are not concerns during RSI because apnea is a desired effect of the process.
Succinylcholine is by far the most commonly used paralytic agent during RSI because of its rapid onset and short duration of action (i.e., approximately 60 seconds and 4 to 5 minutes, respectively). Succinylcholine is a depolarizing neuromuscular blocking agent that acts at the nicotinic acetylcholine receptors at motor endplates. Succinylcholine is metabolized by plasma pseudocholinesterase. Its short duration of action is desirable in the setting of the worst-case cannot-intubate-cannot-ventilate scenario because the patient’s spontaneous ventilations are more likely to return promptly enough to avoid making this failure a terminal event. This agent has an excellent safety profile, but there are potential drug interactions and contraindications to its usage that must be reviewed prior to its use. (See Table 2.)

Succinylcholine also stimulates the muscarinic receptors at the sinoatrial node leading to its negative inotropic and chronotropic characteristics. Bradycardia is a common side effect, particularly in children or when administering repeated doses. Elevation of the serum potassium concentration also occurs, and this effect should be considered prior to use. The administration of succinylcholine to patients with crush or burn injuries and certain musculoskeletal diseases can lead to an accentuated —and potentially fatal— rise in serum potassium levels. There are also several nondepolarizing neuromuscular blocking agents that can be used in RSI and may be chosen when there are contraindications to the administration of succinylcholine. These typically are not used as firstline agents because of their slower onset and longer duration of action when compared with succinylcholine. If succinylcholine must be avoided and a paralytic agent is needed, rocuronium has the shortest onset (i.e., approximately 90 to 120 seconds) but still has a duration of action more than six times that of succinylcholine (more than 30 minutes). Another option is high-dose vecuronium (0.3 mg/kg), whose onset of action is approximately 2 minutes but whose duration of effect is nearly an hour. The nondepolarizing agents are used more commonly as defasiculating agents or to maintain paralysis after the endotracheal tube position has been confirmed.
The proper sequence of medications and timing of events during RSI depend largely upon the medications chosen and the clinical scenario. Ultimately, the goal is to achieve rapid onset of paralysis and sedation while minimizing the risk of aspiration. Equipment setup, patient positioning, and medication choices should all be completed while pre-oxygenating the patient. Once ready, the sedative and paralytic agents should be administered in rapid succession to allow their onset of action to occur nearly in unison. However, sedation should occur immediately prior to paralysis. Cricoid pressure should be applied as the patient begins to lose the ability to protect his own airway. This maneuver will help reduce the risk of passive regurgitation of stomach contents; approximately 8 kg of pressure is required for the maneuver to be effective.12
The loss of airway self-protection typically occurs just prior to the loss of spontaneous respirations, but this can happen at any time after the sedative and/or paralytic agent is given. Unless the patient’s condition and pulmonary reserve are already quite poor, adequate pre-oxygenation should allow the patient to maintain satisfactory oxygen saturation levels while the paralytic and sedative agents take effect and intubation is performed.
Allowing these medications to take full effect prior to laryngoscopy will help increase the likelihood of a successful first attempt at intubation. Avoid providing unnecessary BVM ventilation while awaiting full paralysis; it may increase the risk of aspiration. After the endotracheal tube has been placed into the trachea and its position has been confirmed, cricoid pressure can be released. Early or intermittent release of the cricoid pressure will place the patient at higher risk for aspiration of stomach contents into the trachea. Post-intubation sedative and/or paralytic medications should be chosen and prepared prior to RSI so that they can be given quickly after the endotracheal tube position is verified and before the RSI medications lose effect. Delay in administration can lead to unnecessary patient discomfort and potentially lead to displacement of the endotracheal tube.
There are particular clinical circumstances that arise frequently and warrant special consideration when performing RSI. For example, asthmatic patients may benefit from the bronchodilatory effect of ketamine when used as a sedative agent. Take caution not to inappropriately delay intubation if such a medication is not available readily and the patient requires immediate treatment. The weakened and failing asthmatic patient also may lose respiratory drive and require early assistance if given a defasiculating dose of medication. When pulmonary reserve is severely limited, hypoxia can develop rapidly in spite of attempts at oxygenation. Patients with congestive heart failure and acute pulmonary edema can be difficult to pre-oxygenate and, therefore, will desaturate quickly after given paralytic agents. These patients may require some BVM ventilation prior to laryngoscopy and, therefore, may tolerate only brief attempts at intubation. Patients with suspicion of intracranial or cervical spine injuries will require an extra assistant to hold inline stabilization of the cervical spine once the cervical collar is removed. If left in position during RSI and laryngoscopy, a cervical collar can limit the ability to lift the mandible, thereby, degrading the patient’s maximum achievable laryngoscopic view. Furthermore, the cervical collar is better able to prevent rotation of the cervical spine than extension. Inline stabilization should be performed with the assistant kneeling down to the left of the airway physician while firmly holding the cranium in neutral position without restricting free mobility of the mandible.
Awake Intubation
Awake intubation is an approach that uses medication to facilitate the intubation of a patient who is alert, cooperative, and spontaneously breathing. By avoiding paralysis, this technique preserves the patient’s upper pharyngeal muscle tone to help maintain airway patency and also allows self-protection against aspiration. The approach should be considered for patients with a history of difficult intubation, anticipated airway difficulty, or those with known airway pathology. When using this approach, there is potential for trauma to the airway, laryngospasm, and vomiting. There are four categories of medication that can be used with this approach: sedative, anti-sialogogue, aspiration prophylaxis, and topical anesthetic/vasconstrictor. Many classes of sedative agents exist that can be used cautiously to provide anxiolysis while preserving intact airway reflexes. Aspiration prophylaxis can include non-particulate antacids (e.g., Bicitra and H2 receptor antagonists), and pro-motility agents (e.g., metoclopramide). Topical anesthetic agents offer significant assistance with minimal risk to the patient. Vasoconstricting agents can help greatly if a nasotracheal approach is chosen. Once the patient is prepared with some combination of these medications, any number of airway tools can be utilized, including upright direct laryngoscopic, nasotracheal, and fiberoptic approaches.
The Failed Airway
Failed airway is a broad and imprecise term that can describe multiple airway management challenges. Failed laryngoscopy and the ability to ventilate or not are considered more precise descriptors.
Encountering a patient who neither can be intubated primarily nor ventilated after paralysis and rapidly is becoming more hypoxic (i.e., failed RSI) is one of the most stressful situations a physician can encounter. A practiced approach to this situation should lead to an immediate response. First, the physician should optimize the patient’s airway for BVM ventilation by placing a nasal or oral airway. This approach can make a dramatic difference in the ability to ventilate the patient whose tongue is obstructing the airway in the posterior pharynx. In addition, using a two- or three-person technique for operating the BVM may improve ventilation: One person applies a jaw thrust and seals the mask to the face as tightly as possible; a second compresses the patient’s cheeks anteriorly against the edges of the mask; and a third person solely squeezes the bag. Mastery of the BVM technique often is overlooked when learning how to manage the difficult airway. It may be the last hope for ventilating the patient when all else has failed. Just like many other airway techniques, there exists a wide range of abilities among providers. The time spent improving one’s skill set with the BVM technique is, at least, as valuable as learning a rarely used and highly specialized airway technique.
During laryngoscopy, the BURP technique (i.e., backward, upward, rightward pressure on the cricothyroid cartilage) can be performed to attempt to move the laryngeal structures into a position where they can be visualized. The intubator’s right hand can be placed over the hand of the individual applying cricoid pressure and used to position the larynx in optimal view, which then, can be maintained by the assistant. In some series, this technique has been reported to improve the laryngoscopic view by one Cormack-Lehane grade over the standard anatomic position.13 Additionally, it also can be helpful to reassess the position of the patient’s head; no single position works for all patients. Although the classic sniffing position tends to improve the laryngoscopic view in a majority of patients, there are some patients who require alternate positioning. Should the landmarks not be visualized, a different type or size of blade might improve the view. In some cases where no landmarks are identifiable on laryngoscopy, it may be possible to find the lumen of the airway by pressing on the patient’s chest. This will force air from the lungs to exit through the glottis and a bubble may be seen and could provide the only clue to the approximate location of the glottis. When attempts to visualize the glottis after RSI have failed, the return of spontaneous respirations and muscle tone supporting the airway may allow the vocal cords to come into view. If an esophageal intubation has been recognized, some providers prefer to leave the endotraceal tube (ETT) in the esophagus, which can provide some protection against aspiration and help orient the provider to the location of the glottis.
In some cases, it is possible to locate the epiglottis during laryngoscopy and not visualize the vocal cords when using the Miller blade. This situation can occur when the laryngoscope is off midline. If the laryngoscope is slowly withdrawn and the epiglottis is allowed to fall into resting position, it usually will fall down to the side where the glottis was located and come to rest just in front of the vocal cords. The epiglottis, then, can be lifted again with the tip of the Miller blade while paying attention to better alignment of the instrument.
Finally, if three attempts at direct laryngoscopy have failed, the physician should consider this approach to have failed and move on to one of the rescue techniques (e.g., laryngeal mask airway, Combitube, or cricothyroidotomy) to be discussed in detail later in this article. The authors often consider moving to a backup or rescue device after two attempts in a well-positioned patient who has failed intubation by a skilled airway provider.
Documentation is an area of difficult airway management that often is overlooked. Taking time to describe the areas where difficulty was encountered, the subsequent thought process, and the steps taken to address them is important. Such a note will help those providers who care for the patient after emergency care is rendered. A detailed description can affect the extubation timing and plan. These notes also can clarify reasons for any prolonged efforts or special equipment used.
Tracheal Tube Introducer
The tracheal tube introducer (TTI) is an invaluable adjunct to standard orotracheal intubation. This device commonly, although improperly, is referred to as a bougie because of its origins. In 1942, Dr. MacIntosh from Oxford University used a gum elastic urinary catheter to assist the placement of an endotracheal tube. Current TTIs are constructed of different materials and are not used for dilation as were the original gum elastic bougies. Several variations of the TTI are available with similar designs. These devices are narrow flexible introducers with an angled tip. They are produced as both solid and hollow variations that allow the use of jet ventilation. These devices also can differ by length, material, and degree of flexibility.
The TTI is a unique airway device because of its low cost, practicality, and short learning curve. The authors recommend having a TTI device readily available for every intubation. This device can have excellent success rates when the unexpected difficult airway is encountered with second attempt success rates as high as 80% in anesthetized patients.14 The TTI is an adjunct that can be used when there is a limited view of the larynx during direct laryngoscopy that would have otherwise precluded passage of an endotracheal tube, a situation most often encountered when there is incomplete or no glottic visualization as with Lehane-Cormack modified grade III airway.15 Other situations include those when adequate visualization is achieved (Lehane-Cormack Grade I/II), but the ETT either obstructs the view or is unable to be guided into the trachea. These difficulties tend to occur in patients who have narrow passages or excessive soft tissue in the oral cavity. In these instances, the narrow and maneuverable design of the TTI allows the device to be guided through the vocal cords when the ETT otherwise could not. If the operator is unable to visualize the glottis (Lehane-Cormack Grade IV), then blind passage of the TTI can be attempted. If no visualization is possible, it is recommended that a rescue device be strongly considered early in the course of airway management.
The procedure of orotracheal intubation with the aid of a TTI is intuitive to most providers. Laryngoscopy is initiated in the usual fashion. Rather than passing an ETT through the vocal cords, however, the TTI device is guided through. Holding the device closer to the distal angled tip and feeding it forward allows better control when compared with holding it at the proximal end. In the ideal situation, the TTI would be visualized as it passes through the vocal cords; however, this is not always possible. The anterior angled portion at the tip of the TTI is designed to ride along the undersurface of the epiglottis and move anteriorly into the trachea. When the posterior elements of the glottis (e.g., arytenoids) are visualized, the TTI can be seen to move anterior to these structures after it passes underneath the epiglottis. As the TTI is advanced down the trachea, the cartilaginous rings produce a clicking sensation when the angled tip rides along these structures. This clicking sensation helps to confirm appropriate placement when visualization through the cords is not possible. With further gentle advancement, the operator will feel resistance as its tip runs into either the carina or as it becomes lodged down one of the bronchi. This resistance is a second method that can help confirm correct placement of the TTI. Aggressive and rapid advancement of the TTI potentially could lead to airway trauma and must be avoided. When the device is passed inadvertently into the esophagus, there will be no clicking sensation or resistance during its advancement. Anatomic abnormalities of the esophagus (e.g., strictures or webs) theoretically could lead to false reassurance of correct positioning.
Attention should be paid to the depth of the device once it has been passed down the trachea. Maintaining a minimum depth of 20-23 cm will help ensure that the tip of the TTI remains below the level of the vocal cords. If required at this point during the procedure, the TTI can be moved over to the side of the mouth to allow standard BVM ventilation while taking care not to displace the device from the trachea. Jet ventilation is another option to correct hypoxia if the appropriate type of TTI (e.g., Frova by Cook) was inserted and the appropriate equipment is immediately available and is properly set up. It is vital to ensure that the tip of the TTI is in the trachea prior to any attempt at jet ventilation to minimize barotrauma to the patient. Rupture of a lung can occur easily if the tip of the TTI is placed deeply down into the bronchioles. In addition, jet ventilation equipment can require a significant amount of time to gather and set up if its use was not planned ahead.
Once the TTI is in position, lubrication should be applied to facilitate the passage of an ETT. The ETT, then, is passed in Seldinger fashion over the introducer. The proximal end of the TTI should be held to maintain the device’s linearity to reduce resistance as the tube is advanced. (See Figure 3.) As it is advanced, the ETT’s beveled tip can become caught on various structures, including the base of the tongue, epiglottis, vocal cords, and arytenoid cartilage/folds. Performing a jaw-thrust maneuver using the thumb to grasp the base of the tongue and elevate the mandible or attempting passage with the laryngoscope blade in place will help reduce the chance of encountering resistance from these structures. If resistance is met, the ETT should be withdrawn partially to allow it to disengage from the structure on which it has become caught. The ETT, then, should be rotated 90 degrees to change the orientation of the bevel and re-advanced to allow passage around the obstruction. The TTI, then, should be removed after the ETT is advanced through the vocal cords. Finally, the ETTs position and depth should be confirmed as with any other intubation. There is a tendency for less experienced users to intubate the right mainstem bronchus with this technique because the ETT is inserted too deeply over the TTI.
Backup Approach and Rescue Techniques
If the primary approach to airway management fails, a backup approach should be instituted. Most commonly this occurs after two or three optimized attempts at laryngoscopy. Early recognition of this failure and subsequent transition to a previously developed backup plan can minimize complications. The ability to successfully ventilate the patient after failure of a primary approach will determine which backup techniques are appropriate. If ventilation is difficult or impossible (emergent pathway), a rescue device should be inserted to stabilize the patient. When ventilation is effective and there is sufficient time (non-emergent pathway), it is possible to further attempt intubation with some alternative technique chosen as part of the previously formulated backup plan. Use caution not to attempt complex and time consuming approaches if the primary plan has failed and there is difficulty with ventilation.16
Characteristics of good rescue devices include rapid placement with a high chance of success, mastery without complex training or practice requirements, and the ability to be inserted in a blind fashion. These devices should be used when several attempts at intubation have failed, when no new approaches are appropriate, and when hypoxia is incipient or present. Therefore, they can be used as a primary approach when there is a sudden and unexpected need for airway control in the unstable patient who cannot be ventilated adequately. Typically, this condition is defined as the inability to maintain oxygen saturation level of 90% with proper BVM ventilation.
Rescue devices also can serve as a backup approach in situations when other techniques are unlikely to be successful. Currently, the most common rescue modalities include the laryngeal mask airway, Combitube, and cricothyroidotomy. It is the authors’ opinion that these options —after direct laryngoscopy and RSI— offer the most value to emergency physicians because they are the last line of defense when battling an airway disaster. These techniques will be discussed in detail below.
Laryngeal Mask Airway
The laryngeal mask airway (LMA), invented by Dr. Archie Brain, has proven to be a highly valuable rescue device in the management of the difficult airway since its widespread adoption in the 1990s. The LMA is unique in its ability to function as both a temporary but highly efficacious method of providing oxygenation in the can’t-intubate-can’t-ventilate scenario, as well as an adjunct to definitive blind or fiberoptic-guided endotracheal intubation. A typical scenario in which the physician may chose to use an LMA may occur after several unsuccessful attempts at intubation by direct laryngoscopy or when efforts to mask ventilate the patient between attempts are inadequate in the face of declining oxygen saturation levels.
The LMA consists of an oval inflatable mask affixed to a length of plastic tubing terminating in a standard BVM adaptor. The device is inserted blindly with manual guidance so that the mask seats itself in the larynx in close proximity to the glottic opening. A high success rate for proper placement has been documented, even in novice operators.17
Due to the size of the device, the insertion technique and location when properly positioned, it will act as a rather strong precipitant of the gag reflex. Thus, it is appropriate only for use in patients who are either deeply sedated or comatose.18 The LMA is believed to decrease the risk of gastric insufflation and subsequent aspiration due to its ability to allow adequate ventilation at significantly lower pressures when compared with the typical BVM technique. This is possible due to the LMA’s proximity to the glottic opening. The LMA has been used extensively for general anesthesia in fasting patients in the operating room with an extremely low rate of documented aspiration of 2.6 per 10,000 cases and an acceptably low rate of 11 per 10,000 uses in emergencies.19 Despite these successes, an LMA does not meet the gold standard of airway protection (i.e., a cuffed endotracheal tube properly positioned in the trachea) due to its inability to help shield the lungs from aspiration of gastric contents or pharyngeal secretions. This fact is of particular concern to EM physicians who rarely have the benefit of a patient who has been kept NPO for 8 hours prior to presentation.
The LMA may be of limited assistance to the airway physician in certain situations that will make insertion and positioning difficult (e.g., tight occlusal distance, trismus, angioedema) or will prevent a proper seal from forming (e.g., acquired or congenital structural disease of the larynx, hypopharynx, or glottic opening).
The LMA comes in classic, the slightly improved Pro-Seal, and FastTrach varieties. The FastTrach version has the greatest utility in management of the difficult airway because it is designed to facilitate endotracheal intubation. Modifications that facilitate this goal include a rigid tube with an attached metal handle and a plastic lifting bar suspended from the superior aspect of the mask to elevate the epiglottis out of the path of an endotracheal tube during insertion. A specialized endotracheal tube with an embedded spiral wire to resist kinking is included; in its absence, a standard ETT may be used. A standard ETT should be inserted with the natural curve of the tube opposing the bend of the FastTrach to improve the exit angle from the device. When using a standard ETT, this will allow for easier passage through the vocal cords. The handle permits the operator to manipulate the distal mask portion of the device once properly inserted to help find the best seal and to adjust the position during intubation attempts. This can increase the likelihood of blindly passing an ETT through the lumen of the LMA and then through the vocal cords. The up-down repositioning technique described in the literature involves partially withdrawing then reseating the FastTrach™ to help ensure that the epiglottis is not trapped inferiorly in the path of the ETT.20 The LMA FastTrach delivers the endotracheal tube into the vicinity of the vocal cords, and with trained hands, it produces success rates with blind intubation of nearly 96% within five attempts.21
Should attempts at the blind passage of an ETT fail, the LMA FastTrach provides an excellent conduit to facilitate the use of a fiberoptic scope to guide an ETT through the vocal cords. The specialized endotracheal tube can be loaded onto the fiberoptic scope and then inserted through the LMA FastTrach. Once the trachea has been intubated with the scope, the endotracheal tube can be advanced to follow its path. The use of a fiberoptic scope can be particularly challenging in the emergent setting when attempts to identify laryngeal structures are frustrated by copious secretions and/or blood from multiple previous direct laryngoscopy attempts. Positive pressure ventilation can help improve the fiberoptic view by pushing the soft tissues in the supraglottic region out of the way. To perform this, a side-port adapter must be fitted to the LMA FastTrach to allow positive pressure ventilation while viewing through the fiberoptic scope. It also requires a specialized introducing catheter that will fit over the fiberoptic scope and through the side port adapter. This is clearly an advanced technique that should be performed by an individual with ample experience. The LMA does greatly aid the operator by obviating the need to maneuver the scope to the vicinity of the vocal cords from the oro- or nasopharynx by serving as a passage directly from the mouth to the larynx. In addition, the rigid walls of the LMA FastTrach provide a measure of protection for the scope itself should the patient recover his or her airway reflexes and inadvertently bite down on the fragile and costly fiberoptic scope.
In situations where fiberoptic equipment is not available, the LMA remains a valuable temporizing measure by virtue of allowing rapid insertion with a high probability of achieving successful intubation when other techniques have failed. The patient can be stabilized while preparations are made for alternative but relatively time-consuming techniques for securing the airway (e.g., emergent cricothyroidotomy, retrograde wire intubation, or while the primary operator awaits the arrival of a backup team or additional equipment).
Combitube
The Esophageal-Tracheal Combitube (Tyco-Healthcare-Kendall-Sheridan, Mansfield, MA) is an evolution of the esophageal obturator device previously used by emergency medical services as an alternative to tracheal intubation until the early 1990s. The Combitube is a blindly inserted and pure rescue device for the adult patient. It facilitates adequate ventilation and minimizes the risk of aspiration after attempts at endotracheal intubation have failed. This device represents another valuable addition to the emergency physician’s airway management armamentarium.
The chief advantage of the Combitube is that it is placed using a blind insertion technique. The Combitube is ideal in circumstances when neither the vocal cords nor other glottic landmarks (e.g., arytenoid cartilage and epiglottis) can be identified due to patient anatomy, secretions, or blood in the oropharynx. This is particularly the case when the physician is unable to adequately mask ventilate the patient and a temporizing airway must be established. It can provide adequate ventilation and help stabilize a patient to allow time to plan further attempts at a definitive airway.
The Combitube has enjoyed popularity in the pre-hospital arena due to its ease of use and rapid insertion in situations with limited access to the patient (e.g., during prolonged vehicle extrication) and equipment. In addition, training requirements for the Combitube are modest; novice housestaff operators are able to deliver ventilation within 61 seconds using this device.22
The Combitube consists of a large diameter plastic tube with dual inner lumens and two balloons. It has a distal and smaller esophageal balloon and a proximal larger oropharyngeal balloon. At the proximal end, there are two numbered and color-coded BVM adaptors, one for each lumen in the tube. Lumen number one is blue and terminates proximally at an area where the tube wall is fenestrated between the two balloons. Lumen number two is clear and terminates distally at the end of the Combitube. The Combitube comes in a small adult (SA) variety for patients 4-5½ feet in height, and a standard variety for those taller than 5 feet. There is some overlap between the two sizes, and some authorities recommend using the SA version in most patients shorter than six feet.23
The two lumens of the blindly inserted Combitube help ensure that the physician will be able to ventilate the patient whether the distal portion of the Combitube comes to reside either in the esophagus or the trachea. The Combitube is advanced with manual assistance to allow it to flex around the posterior aspect of the tongue and travel down into the hypopharynx. The tube is in proper position once the front incisors fall between the two black positioning lines on the tube. This should leave the proximal balloon seated just posterior to the hard palate. If difficulty is encountered getting around the bend in the oropharynx, the provider may attempt insertion with a laryngoscope blade in place to elevate the mandible and provide a straighter approach to the hypopharynx.
Once inserted, both balloons are inflated with the recommended amount of air (i.e., 10 cc in the distal balloon and 100 cc or 85 cc in the proximal balloon of the standard and SA varieties, respectively). If the Combitube is placed into the esophagus— as occurs more than 95% of the time— the distal balloon will occlude the esophagus and the proximal balloon will occlude the pharynx.24 If the Combitube was placed into the trachea, the distal balloon will occlude the trachea, and the device will function as an endotracheal tube when the patient is ventilated through the second port.
When ventilation is attempted through port number one and the Combitube is placed in the esophagus, the lungs will be insufflated through the fenestrations in the tube that is positioned near the glottis. If no breath sounds are auscultated and no chest rise is noted, then the tube is in the trachea and ventilation through port two is attempted. End-tidal carbon dioxide (ETCO2) level monitoring also can assist with confirmation of proper positioning. Although counterintuitive, pulling the Combitube back out slightly may improve ventilation when esophageally placed and there is difficult ventilating. When placed too deeply, the walls of the esophagus itself can obstruct the fenestrations.
One of the main benefits of the Combitube over other emergent airway devices is that the device may be left in place for as long as 8 hours, giving the provider adequate opportunity to stabilize the patient and make preparations for placing a definitive airway.25 In the remote likelihood that the Combitube is placed blindly into the trachea, an airway exchange catheter simply may be used to replace the device with an ETT. If the device was placed either in the prehospital setting or in a cannot-intubate-cannot-ventilate situation, further attempts at direct laryngoscopy or nasotracheal fiberoptic intubation can be undertaken with the Combitube still in place. This is performed by deflating the oropharyngeal balloon and shifting the Combitube to one side of the pharynx to improve the physician’s view. Some providers find the Combitube to be bulky and obstructive, but if there is difficulty with visualization of the glottis, the oropharyngeal balloon simply can be re-inflated and ventilation resumed. Some practitioners feel that leaving it in place in the esophagus during attempts at intubation allows some protection of the airway from aspiration. Should efforts at oro- or nasotracheal intubation fail once again, the Combitube may be left in place and used to ventilate the patient during cricothyroidotomy, allowing the operator the relative luxury of performing that procedure under urgent rather than crash circumstances.
Contraindications to the use of a Combitube include obstruction or disease to the larynx or upper esophagus. It should not be used in the alert patient with intact airway reflexes.
The Combitube may provide better protection against aspiration when compared with the LMA because it was designed to rest in the esophagus with an inflated balloon blocking the passive regurgitation of stomach contents. It also may provide the ability to provide higher airway pressure compared with the LMA. Conversion from a Combitube to a standard ETT potentially can be much more difficult than intubation through an LMA, especially when using an intubating LMA or fiberoptic scope. Insertion of the LMA is also somewhat more dependent upon operator technique than the Combitube. It is highly beneficial to practice insertion of the LMA in a controlled setting (e.g., the operating room prior to attempting its use in an emergent situation). Patient size also may limit the usage of a Combitube, whereas there are multiple sizes of LMA available.
Cricothyroidotomy
The need for high airway pressure, long-term airway access, and high risk of aspiration are some factors that may make a surgical approach the favored rescue technique. Additional factors for this approach include upper airway edema, infection, trauma, tumor, foreign body, or chemical/thermal burn to the oropharynx. Cricothyroidotomy is a procedure that is best learned and whose skills are best maintained with hands-on practice. Competence with this procedure and awareness of available equipment are vital for any physician providing airway management. If lacking, these skills should be reviewed and practiced in more detail. High fidelity simulators, task trainers, and animal laboratories all can serve as invaluable tools.
The percutaneous cricothyroidotomy can be performed quickly under the correct conditions. Anatomy can influence the ease of this approach significantly as can the skill of the physician. The surgical cricothyroidotomy typically takes more time, but does allow for visualization of structures even when the anatomy of the neck is distorted (e.g., in patients with edema or in obese patients). In a crash situation, the skin incision should be made vertically, which will allow for extension of the incision and better exposure when the anatomy is challenging. The vertical incision is less likely to cause injury to lateral neurovascular structures and typically produces less bleeding. Either of these techniques can be performed in a much more comfortable setting when the tenuous patient can be oxygenated with a temporizing device such as an LMA or a Combitube in place.
Sudden Loss of Airway
There are instances in which the sudden and unexpected need for airway control arise. These can be challenging because the ability to plan and prepare have been taken away. BVM ventilation should be the firstline of treatment in this situation. This ideally will provide oxygen to the patient while equipment and assistance are gathered. This simple step will help determine which subsequent airway management options are viable. If the patient cannot be ventilated with a BVM, the provider immediately should institute the emergency airway pathway and utilize a rescue device. When the patient is able to be ventilated and maintain oxygen saturation levels above 90%, there is time to perform further assessment and develop a management plan. An operator who defers attempts at ventilating a patient who is becoming increasingly hypoxic and bradycardic does so at his or her own peril; cardiac arrest in no way simplifies the task of securing the airway.
While physicians devote considerable effort and attention to difficulties involved in securing the airway of a patient in respiratory failure, a situation in which the intubated and sedated patient can no longer be ventilated adequately can be a similarly perilous occurrence. The approach to this topic often receives short shrift in discussions of airway management among emergency physicians; yet with increasing emergency department length of stay of admitted patients, it is a problem that providers increasingly seem likely to encounter.
In these situations, the physician often will be summoned to the bedside of a ventilated patient who is found to be frankly hypoxic or worse: bradycardic and hemodynamically unstable. There are four main categories to consider as the cause when unable to ventilate an intubated patient: intrinsic airway, pulmonary issues, central causes, and equipment related. Intrinsic airway relates to either dislodgement or obstruction of the endotracheal tube. These can be due to mucous plugging or aspirated debris, particularly in patients intubated due to respiratory failure secondary to pneumonia, chronic obstructive pulmonary disease, or aspiration pneumonitis. The second category of causes is related to pulmonary issues and includes worsening of the primary respiratory failure process (e.g., congestive heart failure and pneumonia), which increasingly may require higher Fi02 or positive end-expiratory pressure (PEEP) to adequately oxygenate the patient. Another life-threatening pulmonary issue that must be considered is a tension pneumothorax. Pneumothoraces may occur spontaneously in patients with pulmonary blebs due to emphysema. A preexisting pneumothorax may have occurred in the initial injury of a trauma patient, but may not have been apparent either clinically or on initial plain radiographs. In either case, what might be a clinically insignificant pneumothorax in a patient with spontaneous ventilations quickly can transform into a tension pneumothorax when a patient is provided positive pressure ventilation. A third category of central causes relates to difficulties regarding inadequate sedation in the nonparalyzed patient who is fighting the ventilator. In the final category, one should consider malfunction of the ventilator itself or a leaking or disconnected oxygen tubing circuit.
The initial physician response to this situation should begin with an attempt to verify the ETT position and lung expansion by auscultation. The presence of breath sounds on both sides of the chest as well as quantitative (preferred) or qualitative measurement of end-tidal CO2 should be determined. The physician should remove any unstable patient from the ventilator and ventilate the patient with a BVM with 100% oxygen. This allows the physician to eliminate equipment malfunction as a cause while at the same time assessing how much effort is required to inflate the lungs (compliance) to assess for the possibility of hemo/pneumothorax as a contributor. In some cases (e.g., in cardiac arrest) the ETCO2 level may not be a good predictor of proper tube placement and other alternatives (e.g., an esophageal detector device [EDD] or fiberoptic scope) should be considered. Additionally, the physician can attempt to pass an in-line suction catheter down the endotracheal tube to remove any mucous plugs or aspirated debris that might be causing obstruction.
Equipment and Additional Techniques
Airway equipment may be stored and organized in either a unified or split arrangement. The unified cart or bag stores all of the emergency department’s airway equipment—both routine and difficult—together in one place. The alternative is to rely upon the standardized assortment of ETTs, blades, and handles in the hospital-wide code cart while maintaining an individualized difficult airway bag or cart for specialized equipment. A more portable bag or tackle box setup may be particularly helpful for emergency physicians who respond to codes elsewhere in the hospital or department on a regular basis. Regardless of the arrangement chosen, all airway equipment should be stored in a constant location and maintained in good working order with regular restocking. It is unacceptable to waste time searching for a device when providing care to a patient in acute need of airway control.
There are numerous devices and techniques that can be used in specialized circumstances. Some of these devices tend to be of lower yield to the average practicing emergency physician because they are more complicated to learn and require continued practice to maintain competency. These other devices also tend to be used much less frequently than some of the rescue devices mentioned earlier in this article. This section focuses on some additional equipment including devices to confirm correct endotracheal tube placement. A selection of additional devices and techniques are mentioned briefly below.
Endotracheal Tube Position Verification
Unrecognized esophageal intubation is one of the most catastrophic complications of attempted endotracheal intubation. For this reason, measurement of end-tidal CO2 return immediately post intubation —in addition to auscultation and subsequent chest radiograph— is of particular importance in management of the difficult airway when the physician may be forced to place an endotracheal tube without direct visualization of the vocal cords. (See TTI, LMA and Combitube© sections above.) While qualitative methods (e.g., the EasyCap colorimetric device) are relatively inexpensive and convenient, quantitative devices allow continuous monitoring and provide a wealth of useful information. For added reassurance, the end-tidal measurement may be observed during the first 30 seconds after intubation to confirm that the tube is indeed in the trachea. The small amount of CO2 present in the stomach from insufflation with exhaled gases during mask ventilation will dissipate by the sixth breath, while breath-to-breath end -idal CO2 is quite consistent.2 The end-tidal CO2 level has a direct correlation with the PCO2 level—A 2-5 mmHg gradient is average, but it may be up to 15 mmHg in those patients with severe lung disease— which can allow bedside assessment of the adequacy of ventilation of a patient on a respirator, while oximetry indicates proper oxygenation.26 End -idal CO2 levels also have been shown to be of prognostic value in CPR, with no patient surviving cardiac arrest in two separate studies with an average end-tidal CO2 level less than 10 mmHG during CPR.27 A rapid rise in measured end -idal CO2 levels during CPR often signifies the pronounced increase in cardiac output that accompanies the return of spontaneous circulation.28 As helpful as capnography is, clinicians should continue to use other methods of verifying tracheal tube placement such as auscultation of lung and gastric sounds, observation of tube condensation, or presence and presence of gastric contents in tube; no single method is infallible.29
Another excellent modality for ascertaining whether an endotracheal tube has been placed in the trachea or the esophagus is the esophageal detector device (EDD). The most common version consists of a self-inflating deformable rubber or plastic bulb attached to a standard endotracheal tube adaptor. The EDD’s bulb is compressed before it is attached to the ETT, thus, applying suction to the lumen of the ETT. This device capitalizes on the difference between these two anatomical structures; suction applied to a tube placed in the esophagus will collapse its soft deformable walls and prevent the bulb from reinflating. The trachea’s cartilage rings will maintain patency of the lumen and allow the bulb to re-expand as air is drawn in from the patent trachea through the ETT. While the EDD has been ignored by many providers since the advent of end-tidal CO2 detection, it retains an advantage in certain common situations (e.g., severe pulmonary edema, cardiac arrest) that reduce the amount of CO2 detectable by capnography owing either to decreased CO2 production or increasing ventilation-perfusion mismatch.30 Some prospective series have shown favorable performance for the EDD compared with end-tidal CO2 measurement (99% vs 87% sensitivity, respectively) in clinical use.31
Blind Nasotracheal Intubation
Nasotracheal intubation remains an option worthy of consideration in situations where the physician would prefer to secure the airway with the patient awake and breathing spontaneously while avoiding paralytic agents. This might be the case in a patient with an anticipated difficult airway or with contraindications to using succinylcholine (e.g., hyperkalemia). This technique is not appropriate in trauma patients with suspected cranial or midface injuries due to the risk of false passage of the tube into soft tissue or worse, the calvarium itself. Patients who are anticoagulated are also poor candidates due to the risk of significant hemorrhage. Mucosal injury that may occur during attempts to introduce the tube into the nasopharynx can lead to hemorrhage; up to 69% of patients may experience epistaxis.32 The use of topical vasoconstrictors (e.g., Afrin, Neo-synephrine) several minutes prior can help shrink the nasal mucosa to avoid injury and improve ease of tube passage. Topical analgesia with lidocaine jelly can both improve patient comfort and act as a lubricant for tube passage. This technique has a significantly lower rate of success than direct laryngoscopy, with rates between 85% and 68%.19,33 The directional tip endotracheal tube (Endotrol, Mallinckrodt Medical Incorporated, St. Louis, MO) or trigger tube, designed specifically for nasotracheal intubation, allows the operator to direct the tip of the tube anteriorly to facilitate passage into larynx rather than the esophagus. This device was associated with a significant increase in success rate (58% vs 72%) in a study involving paramedics.34
Transtracheal Jet Ventilation
The transtracheal jet ventilation (TTJV) is a semi-invasive technique in which a large bore needle is placed through the cricothyroid membrane and high pressure oxygen is delivered through a specialized setup of tubing, filter, and valve. The pressurized oxygen fills the lungs and then exits passively through the trachea; therefore, a patent airway must be present. Caution and experience are important to prevent barotrauma. Stacking breaths without allowing adequate time for escape of the gas can lead to the rupture of a lung and subsequent tension pneumothorax. Although a temporizing technique, it can provide excellent oxygenation while other attempts at airway control are made.
Inverse Intubation
Inverse intubation is an interesting technique in which laryngoscopy is performed from the opposite side of the patient (i.e., below the patient’s head) and with the laryngoscope in the right hand and held upside down. These are all opposite of the usual laryngoscopic technique in which the operator stands above the patient’s head and holds the laryngoscope in the left hand with the blade held below the hand. This technique can be performed in a one-person or a two-person fashion and offers some mechanical advantage (i.e., pulling rather than pushing). Direct observation of the laryngoscope blade as it passes through the oral cavity and into the vallecula is recommended to avoid pharyngeal trauma including laceration.35
Retrograde Wire
The retrograde wire technique is used rarely, but may have a role in the emergency department in patients with significant upper airway deformation related either to neoplasm, maxillofacial trauma, or congenital abnormalities. This technique involves placing a needle through the cricothyroid membrane and then, percutaneously feeding a wire superiorly through the vocal cords. The wire then is advanced into the pharynx where it can act as a guidewire for the insertion of either an endotracheal tube or a fiberoptic scope—with the wire passing through the suction port—through the vocal cords. A recent innovation involves use of a flexible plastic sheath similar to a tube exchange catheter, which is advanced over the wire to the very end coming to rest at the cricothyroid membrane. Then, the wire is withdrawn, and the sheath is allowed to advance through the vocal cords. An endotracheal tube is advanced over this plastic sheath and directly through the vocal cords. This modification is believed to reduce the chance of one complication of this procedure: kinking of the endotracheal tube on the wire while in the airway above the vocal cords.36 This technique is relatively time consuming, therefore, it is best suited for patients who can be ventilated during the procedure. Commercially available kits contain this specialized equipment in an organized fashion; use of commonly available sterile wires from central venous access kits is ill advised due to their shorter length, smaller diameter, and resultant lack of sufficient rigidity. If possible, this procedure should be avoided in patients with anticoagulation or a bleeding diathesis. Although this technique can be performed fairly quickly in experienced hands, it can be cumbersome and frustrating in a panic situation.
Lighted Stylet
Lighted stylets facilitate a blind intubation by capitalizing on the fact that a light source at the tip of a malleable stylet placed in the anterior trachea or glottis will transilluminate the soft tissues of the neck. When the same source is placed posteriorly in the esophagus, it will not be visible as a focused spot of light.
While the technique has been described as early as 1959, newer devices such as the Trachlight (Laerdal Medical Corporation, New York, USA) have shown success rates in excess of 99% in some series, with a 92% first-attempt intubation rate.37 The Trachlight has a brighter light source than previous devices, adapts to different sizes and lengths of standard endotracheal tubes, has a light wand flexible enough to allow nasotracheal insertion, and is reusable. In addition, the stylet is retractable to minimize trauma during passage of the tube through the vocal cords. There are several significant contraindications to this technique, which include awake and noncompliant patients, obese patients (whose necks will be difficult to transilluminate), or in the setting of laryngeal trauma, a neoplasm, or a foreign body.38 Although the risk of laryngeal trauma is quite low in the hands of a skilled operator, caution should be used in anticoagulated patients.39 This technique, while simple in principle, has a steep learning curve because of the need to be able to infer the position of the tip of the endotracheal tube based on subtle changes in the transillumination of the neck.
Conclusion
There is a wide array of airway management equipment on the market and a continuing procession of new devices that frankly can be overwhelming to clinicians who are attempting to define an approach to difficult airway management. What is most important based upon the authors’ experience is for clinicians to establish a thorough understanding as well as a hands-on familiarity with a small, but sufficient, number of rescue devices and techniques. No piece of equipment can be used with skill or facility on the first attempt—they come with practice. In addition to learning a select number of devices, physicians also must seek opportunities to develop and maintain their skills. The incorporation of newer devices and techniques into the practicing physicians repertoire can be challenging. Clinical practice, advanced patient simulators, and static trainers all can provide the resources to develop these skills. A well-intended difficult airway course unfortunately can lead to a short-lived and superficial capability with complicated devices. A true level of comfort and ability may be obtained from repetitive practicing with a single device in varying clinical scenarios. Some devices (e.g., the Combitube) may be less suited for practice in actual clinical care. With advancing technology, airway simulators are better able to provide a realistic environment in which to master these skills.
References
1. Bair AE, Filbin MR, Kulkami RG, et al. The failed intubation attempt in the emergency department: Analysis of prevalence, rescue techniques, and personnel. J Emerg Med 2002:23;131-140.
2. Orebaugh, SL. Difficult airway management in the emergency department. J Emerg Med 2002:22;31-48.
3. Ezri T, et al. Recent trends in tracheal intubation: Emphasis on the difficult airway. Curr Opin Anaesthesiology 2004:17; 487-490.
4. Levitan RM, Everett WW, Ochroch, EA. Limitations of difficult airway prediction in patients intubated in the emergency department. Ann Emerg Med 2004:44;307-313.
5. Behringer, EC. Approaches to managing the upper airway. Anesthesiol Clin North Am 2002:20;813-832.
6. Butler KH, Clyne B. Management of the difficult airway: Alternative airway techniques and adjuncts. Emerg Med Clin North Am 2003; 21: 259-289.
7. Marvez, E, Weiss, S, Houry DE, et al. Predicting adverse outcomes in a diagnosis-based protocol system for rapid sequence intubation. Am J Emerg Med 2003:21;23-29.
8. Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg 2004;99:607-613.
9. Sackles, JC. Airway management in the emergency department. Ann Emerg Med 1998:31:3.
10. Bushra JS, McNeil B, Wald DA. A comparison of trauma intubations managed by anesthesiologists and emergency physicians. Acad Emerg Med 2004;11:66-70.
11. Lev R, Rosen P. Prophylactic lidocaine use preintubation: A review. J Emerg Med 1994;12:499-506.
12. Jackson WL. Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock? Chest 2005; 127:1031-1038.
13. Hartmannsgruber MW, Gabrielli A, Layona J, et al. The traumatic airway: The anesthesiologist’s role in the emergency room. Internat Anesthesiol Clin 2000:38;87-104.
14. Benumof JL. Quantitative improvement in laryngoscopic view by optimal external laryngeal manipulation. J Clin Anesthesia 1996;8:136-140.
15. Combes X, Le Roux B, Suen P, et al.Uunanticipated difficult airway in anesthetized patients. Anesthesiology 2004;100:1146-1150.
16. Hames KC. Use of the Bougie in simulated difficult intubation. Anesthesia 2003:58; 846-851.
17. Levitan RM. Patient safety in emergency airway management and rapid sequence intubation: Metaphorical lessons from skydiving. Ann Emerg Med 2003;42:81-87.
18. Yardy N, Hancox D, Strang T. A comparison of two airway aids for emergency use by unskilled personnel. Anaesthesia 1999;54:181-183.
19. Reardon RF. The intubating laryngeal mask airway. Acad Emerg Med 2004; 8:833-837.
20. Harry RM. The use of cricoid pressure with the intubating laryngeal mask. Anaesthesia 1999;54:656-659.
21. Ferson DZ, Rosenblatt WH, Johansen MJ. Use of the intubating LMA-Fastrach in 254 Patients with difficult-to-manage airways. Anesthesiology 2001; 95:1175-1181.
22. Dorges V, Ocker H, Wenzel V. Emergency airway management by non-anesthesia house officers. Emerg Med J 2001;18:90-94.
23. Levitan RM, Frass M. The Combitube as rescue device: Recommended use of the small adult size for all patients six feet tall or shorter. Ann Emerg Med 2004;44:92-93.
24. Agro F, Frass M, Benumof JL. Current status of the combitube. J Clin Anesthesia 2002;14:307-314.
25. Linko K, Paloheimo M, Tammisto T. Capnography for detection of accidental oesophageal intubation. Acta Anaesthesiol Scand 1983;27:199-202.
26. Marx J. Rosen’s Emergency Medicine: Concepts and Clinical Practice, 5th ed. Mosby, Inc:2002:32
27. Sanders AB, Kern KB, Otto CW, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation: A prognostic indicator for survival. JAMA 1989;262:1347.
28. Wayne MA, Levine RL, Miller CC. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med 1995;25:762.
29. Garnett AR, Ornato JP, Gonzalez ER. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA 1987; 257:512-515.
30. Li J. Capnography alone is imperfect for endotracheal tube placement confirmation during emergency intubation. J Emerg Med 2001;20:223-229.
31. Salem MR. Verification of endotracheal tube position. Anesthesiol Clin North Am 2001; 4:813-839.
32. Bozeman WP, Hexter D, Liang HK, et al. Esophageal detector device versus detection of end-tidal carbon dioxide level in emergency intubation. Ann Emerg Med 1996; 27: 595-599.
33. Dronen SC, Merigian KS, Hedges JR, et al. A comparison of blind nasotracheal and succinylcholine-assisted intubation. Ann Emerg Med 1987;16: 650-652.
34. O’Connor RE, Megargel RE, Schnyder ME. Paramedic success rate for blind nasotracheal intubation is improved with the directional tip control. Ann Emerg Med 2000;36:328-332.
35. Smally AJ, Dufel S, Beckham J, et al. Inverse intubation: Potential for complications [Brief Report]. J Trauma 2002;52:1005-1007.
36. Wijesinghe HS, Gough JE. Complications of a retrograde intubation in a trauma patient. Acad Emerg Med 2000;11:1267-1270.
37. Hung OR, Pytka S, Morris L, et al. Clinical trial of a new lightwand device (Trachlight) to intubate the trachea. Anesthesiology 1995;83:509-514.
38. Agro F, Hung OR, Cataldo R, et al. Lightwand intubation using the Trachlight: A brief review of current knowledge. Can J Anaesth 2001;48:592-599.
39. Hung OR, Pytka S, Morris L, et al. Lightwand intubation: II - Clinical trial of a new lightwand for tracheal intubation in patients with difficult airways. Can. J Anaesth 1995;42:826–830.
