Food Allergy
Author: Jonathan Glauser, MD, FACEP, Associate Chair, Operations, Department of Emergency Medicine, Cleveland Clinic Foundation; Faculty, Residency Program in Emergency Medicine, MetroHealth Medical Center, Cleveland, OH.
Peer Reviewers: J. Stephan Stapczynski, MD, Chair, Emergency Medicine Department, Maricopa Medical Center, Phoenix, AZ; and Stephen Crabtree, DO, FACEP, Associate Professor of Emergency Medicine, Medical College of Georgia, Augusta.
Recent epidemiologic studies indicate that nearly 4% of Americans are afflicted with food allergies. This includes from 5-8% of children younger than 4 years of age1 and 1-2% of adults.2,3,4 (See Table 1.) The prevalence of peanut allergy alone appears to have doubled in American children younger than 5 years of age in the past 5 years.4 Food allergy is the leading cause of anaphylaxis treated in the United States. It accounts for approximately 30,000 anaphylactic reactions, 2000 hospitalizations, and 150-200 deaths annually in this country.5 As many as 30% of American adults have self-reported food allergy, altering their eating habits accordingly.6
Table 1.
Prevalence of Food Allergies
in the United States2-4

Food allergens are almost always proteins. A food allergen has the ability first to elicit an IgE response, and then on subsequent exposure, to elicit a clinical response to the same protein. There are unanswered questions as to what biochemical properties are required for a protein to survive food processing, to escape the digestive enzymes of the human gastrointestinal tract, and interact with the immune system. An abundance of protein in the food as well as the presence of linear IgE binding epitopes on those proteins play a role.7 The spectrum of food allergy ranges from atopic dermatitis or other cutaneous manifestations hours after eating the problem food, to life-threatening events occurring shortly after ingestion. As well, some individuals experience allergic symptoms only if the food is eaten before physical stimuli such as vigorous physical exercise. —The Editor
Epidemiology
People who have life-threatening reactions to food usually have asthma and often have a history of atopy.8,9 They may have atopic dermatitis or a history of food allergy as young children, and typically are young—ages 11-21 years.10 Very often there is a history of a prior reaction to the same food, albeit milder. The incidence of food allergy is highest in the first two years of life and declines with age. Foods introduced during the first two years of life, therefore, appear most likely to induce hypersensitivity reactions, with cow’s milk, eggs, soy, wheat, and peanuts among these. The entire list of foods associated with anaphylaxis is more extensive. (See Table 2.) There are well-described cases of patients experiencing catastrophic anaphylactic reactions to foods as teenagers when they had minimal gastrointestinal symptoms as a young child.
Table 2.
Foods Reported to be Associated
with Food Anaphylaxis

Peanut allergy has shown a rising prevalence in westernized countries. The causes for this may be several: demand for highly nutritious "quick-energy" foods that has made the peanut popular in the American diet, as well as the popularity of peanuts as nutritional sources for pregnant or lactating women.
The development and expression of atopic diseases is felt to depend upon a complex interaction between genetic factors, environmental exposure to allergens, and other factors, including tobacco smoke, air pollution, and infections. For example, the per capita consumption of peanuts in China and the United States is virtually the same, but peanut allergy in China essentially does not exist. Since people in America of Chinese descent have an incidence of peanut allergy similar to Americans in general, peanut allergy has been attributed more to the roasting and manufacturing process in this country than to any particular genetic makeup.11 Early dietary exposure undoubtedly plays a role in later food tolerance. In one report, infants fed exclusively breast milk or extensively hydrolyzed formula for at least four months experienced a lower incidence of cow’s milk allergy.12
Pathophysiology
The development of an IgE-mediated reaction to an allergen is a result of a series of interactions involving antigen-producing cells, T cells and B cells. The process results in the production of antigen-specific IgE, which circulates in the bloodstream.13 Food allergy represents an abnormal response to antigens delivered orally, and largely may be a reflection of developmental immaturity of various components of the gut barrier. For example, adaptive immune responses are not well-developed in the intestines of infants, nor is enzymatic activity in the newborn period. This immature state of the mucosal barrier might play a role in the increased prevalence of gastrointestinal infections and food allergy seen in the first years of life.14
Anaphylactic reactions require previous exposure for sensitization. Later, if contact with an allergen occurs, bridging occurs between molecules of specific IgE molecules on the cell surface, causing the release of mediators and cytokines from mast cells and basophils. Mediators such as histamine, tryptase, leukotrienes, eosinophil cationic protein (ECP), and other eosinophil activation markers (EDN) are released and may be detectable on laboratory screening.
IgE antibodies do not cross the placenta. For an infant to show a reaction to peanuts, for example, suggests that peanut protein is encountered in utero or via breast milk.15 In one study of 54 infants younger than 11 days of age and another 71 who were younger than 4 months of age, 8% had a positive skin-prick test for peanuts.16
In the United States, the majority of children have been exposed to peanuts or peanut butter by their second birthday.17 There is a much higher incidence of exposure than in many European countries. The method for preparing and cooking peanuts affects the prevalence of allergy as well. Most peanuts in the United States are dry-roasted, including those used to make peanut butter, whereas peanuts in China are typically boiled or fried. The higher temperatures required for dry roasting increase the allergenicity of the three major peanut proteins more than do the lower temperatures used for boiling of frying.18 While genetics may play a role, the prevalence of peanut allergy is low in China, but similar among the children of Chinese immigrants to the United States and the children of native-born Americans.19
Food sensitization may occur following an unknown exposure to a food antigen, as when a baby sitter or grandparent gives a milk formula. Food may be contained in a product not suspected of containing the antigen in question. It has been proposed that sensitization may occur in utero.20,21
Symptoms
Some symptoms of food-induced anaphylaxis will occur within 5-20 minutes of ingestion of a food allergen. In general, the later the onset of symptoms after ingestion, the less severe the reaction will be.22 Early symptoms of food-induced anaphylaxis may include oral tingling, pharyngeal pruritus, and a feeling of tightening of the airways. Gastrointestinal symptoms may include abdominal cramping, nausea, and vomiting. Diarrhea or colic may be present. Dermatologic signs may include urticaria, angioedema, and cutaneous flushing.13
Fatal and non-fatal cases may exhibit hypotension, progressive respiratory distress, respiratory obstruction, dysrhythmias, and cardiovascular collapse.22 Hypotension may be a result of asphyxia. Chest pain may occur and may signify cardiac ischemia. Food-associated exercise-induced anaphylaxis is a form of anaphylaxis that occurs within 2-4 hours of ingesting a food. In this disorder, the food may be ingested safely in the absence of exercise.23
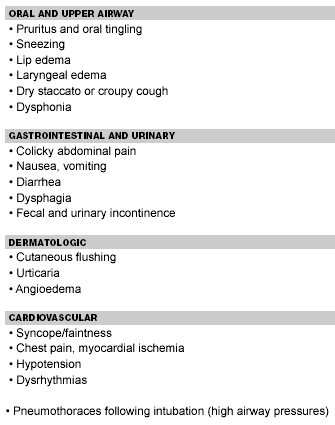
Biphasic reactions have been noted in up to one-third of patients with near-fatal anaphylaxis. These patients apparently recover from the initial reaction when bronchospasm recurs, typically 1.5-3 hours after the initial reaction, although a delay of up to 72 hours has been reported.24,10 Intubation and mechanical ventilation may be required. Secondary pneumothoraces are not uncommon as a result of the high pressures required to ventilate. Patients may have protracted symptoms lasting up to three weeks. There is some evidence that early epinephrine administration may affect the prevalence of biphasic or protracted symptoms, but that steroid administration does not.22 Table 3 lists symptoms of food-induced anaphylaxis.
Table 3.
Symptoms of Food-Induced Anaphylaxis
Food hypersensitivity disorders may cause a variety of gastro-intestinal disorders. Allergic eosinophilic esophagitis is seen typically from infancy until adolescence and presents with symptoms of reflux, dysphagia, abdominal pain, and vomiting. Diagnosis is by endoscopy and biopsy.25 Allergic eosinophilic gastroenteritis can occur at any age, presenting with postprandial projectile emesis, mimicking pyloric stenosis in the infant. Vomiting, diarrhea, anemia, weight loss, or failure to thrive in infants are hallmarks of this disorder.26,27 Food protein-induced enterocolitis is another eosinophilic disorder diagnosed endoscopically with biopsy. Infants may be otherwise healthy, but present with gross or microscopic blood in the stool. Lesions are confined to the distal large bowel. The disorder appears to be caused by food proteins passed in maternal breast milk or milk- or soy-based formulas.28
Respiratory symptoms from food allergy include asthma as well as anaphylaxis. Heiner’s syndrome is a rare form of pulmonary hemosiderosis related to cow’s milk ingestion.29
Chronic urticaria and angioedema may occur as well, with symptoms lasting more than six weeks.30 Atopic dermatitis may present as a chronic eczematous syndrome. Dermatitis herpetiformis is a chronic blistering disorder affecting extensor surfaces and buttocks typically, associated with gluten-sensitive enteropathy.31
Diagnosis and Laboratory Evaluation
The diagnosis of an acute allergic reaction is based upon clinical symptoms and a history of exposure to an allergen. Specifically, the detection of food-specific IgE and a history compatible with IgE-mediated symptoms occurring within one hour of ingestion of the food in question is required. A food diary may help elucidate the offending agent. For peanut allergy, the most common cause for food-induced anaphylaxis and death in this country, the history of exposure may not be present. Peanuts or peanut butter frequently are added to candy, cookies, pastries, and gravies. Peanut butter may be used in Chinese restaurants to hold together overlapping edges of egg rolls or used to cook a variety of meals in the same wok.
Several assays exist for chemical markers of anaphylaxis. Serum beta-tryptase levels are a hallmark of mast cell activation which, measured within four hours, may be elevated with anaphylactic reactions. However, beta-tryptase levels usually remain normal in food-induced anaphylaxis,32 and rarely are available in the emergency setting. Plasma histamine levels may be elevated only approximately 43% of the time.33 The level of plasma histamine rises dramatically, but the peak lasts only for a few minutes.24 Serum eosinophil cationic protein (ECP) and urinary eosinophil activation markers (EDN) also are measures of chemical mediators of IgE-mediated allergy which seldom, if ever, have clinical utility in the emergency setting. ECP has been noted to be elevated during exercise-induced anaphylaxis.24 Approximately one-half of patients with eosinophilic gastro-enteritis or esophagitis have peripheral eosinophilia and may have anemia or decreased serum protein and albumin.28,4 Total serum IgE may be elevated in allergy, but normal values do not exclude allergy, and other conditions can cause elevated levels.34 Screening for specific anti-gliadin IgG and IgA may be needed for the diagnosis of wheat flour allergy or celiac disease.
The laboratory evaluation of food-induced anaphylaxis generally is focused on the identification of specific IgE antibodies to the suspected food. Accepted testing for the presence of IgE centers on skin prick testing and on radioallergosorbent (RAST) tests. Specific categories of tests are discussed in turn.
Prick/puncture skin testing is widely used. Skin prick tests are inexpensive and may be performed in individuals of all ages. A negative prick/puncture skin test is an excellent predictor (greater than 95%) of a negative IgE-mediated food reaction to that particular food.4 False negative tests may result if the patient is taking an anti-histamine, if the food extracts used have inadequate allergenic potential, or if improper technique is used. A positive skin test is defined as 3 mm or greater than the negative control. Strongly positive results, as manifested by a wheal of more than 8-10 mm diameter, indicate a greater likelihood of clinical reactivity. A patient with a positive skin test but a questionable clinical history should undergo a food challenge to clarify the diagnosis.13 Commercially available extracts are not standardized, and potency varies by manufacturer and by food.34
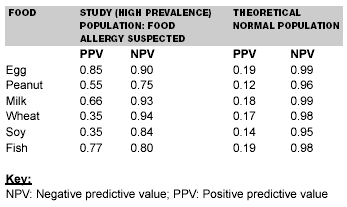
Patients with generalized atopic dermatitis may make skin prick testing impossible. However, a positive skin prick test does not necessarily mean that the patient will react to that food. Skin prick tests exhibit a low specificity: The positive predictive value of an isolated skin prick test is less than 50%.35 If there is a strong suspicion that a food may have precipitated an anaphylactic reaction despite a negative skin prick test, the patient may be tested with the natural food utilizing a prick plus prick test to ensure the absence of detectable IgE antibody.36 Multi-detection assays may include as many as 30 food allergens. It is notable that cross reactions are not infrequent, as among latex, fruits, and vegetables.37,38 Positive and negative predictive values of skin prick testing have been studied for a variety of antigens.39 (See Table 4.)
Table 4.
Positive and Negative Predictive Value
of Skin Prick Test for Specific Foods39
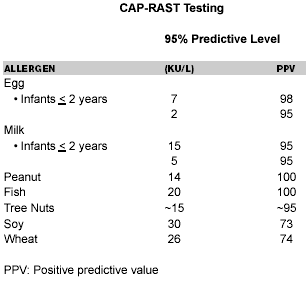
Radioallergosorbent testing (RAST) involves food-specific testing for absolute values of IgE. Production of IgE antibodies is characteristic of atopy, and the determination of specific IgE serum levels against various allergens is an essential element in the diagnosis of sensitization. High levels of IgE to egg (> 6 kIU/L), peanut (> 15 kIU/L), fish (>20 kIU/L), and milk (> 32 kIU/L) reach a 95% positive predictive value.38 Serum-specific IgE appears to be a sensitive measure for resolution of food allergy.40 This testing is particularly useful in patients who cannot discontinue anti-histamines because of severe allergic symptoms, in patients with severe atopic dermatitis or dermatographism, and in adults with known anaphylaxis to a food. Sensitivity and specificity for CAP-RAST testing vary by food and by age of the patient.41 Pharmacia-CAP-RAST studies have shown a 90% or greater predictive value for four foods: milk, egg, peanut, and fish. (See Table 5.)42,43,44,45,4 Quantitative measurements of food-specific IgE antibodies may be relied upon more in the future in predicting and diagnosing IgE food allergy.4
Table 5.
Predictive Value of Food
Allergen-Specific IgE Levels4,42-45
Double-blind placebo-controlled food challenges (DBPCFC) may be needed to identify the responsible food if several foods were ingested and the patient has positive skin tests to several foods. DBPCFC is considered the gold standard of food allergy diagnosis and requires the administration of the food in question to the patient in a blinded, controlled setting.46 The safety of the procedure has been described.47 Similarly, an oral food challenge may be warranted under physician supervision following an extended period of food elimination to verify that a person has outgrown his or her reactivity to peanuts, tree nuts, or shellfish. Choosing a placebo that can completely mask the test food can be difficult. As well, the challenge material should be administered in a few gradually increasing doses, starting with a safe dose for a particular patient.48,49 When the history and laboratory tests give a convincing diagnosis of a specific food allergy, DBPCFC is not indicated. Where there is a clear history of anaphylaxis, oral food challenge is relatively contraindicated. Nonetheless, if there is a suspicion of allergic reactivity to a food in spite of a negative skin prick test, physician-supervised food challenge may be necessary to confirm the absence of a particular allergy. Suspect foods should be eliminated prior to testing for 7-14 days and longer if there is a non-IgE-mediated gastrointestinal disorder such as eosinophilic gastroenteritis.4
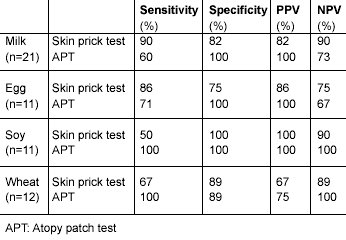
Food patch testing frequently is done in patients with atopic dermatitis. Patch tests reflect T-cell mediated reactions and correlate with relevant foods as confirmed by food challenges. Positive and negative predictive values have been generated for atopy patch testing for a variety of antigens. (See Table 6.)50,51,52 Atopy patch tests may be helpful in evaluating some patients with eosinophilic esophagitis as well—a disorder characterized by eosinophilic infiltration of the esophagus, which is T-cell mediated, presenting with achalasia, failure to thrive, or esophageal reflux refractory to therapy. Food proteins are dissolved in saline and placed in discs that are left on the skin for 48 hours.13 Food allergy has been divided into primary food allergy, which affects young children (class I), while adults frequently develop food allergy as a result of inhalant sensitization (class II). Skin tests have been criticized because they depend on food extracts, which differ in their content of individual allergens, and vary among different manufacturers and different batches.53
Table 6.
Predictive Values for Atopy Patch Test and Skin Test50-52
The presence of food-specific IgE antibodies does not always correlate with clinical symptoms against the respective food. Oral provocation tests, therefore, need to be performed to validate serological diagnosis or skin tests. While DBPCFC is desirable, open non-blinded challenges may be acceptable if performed in an appropriate medical setting with resuscitation facilities available.13
Specific Allergies
Most people who have significant food allergies develop them to one of only a handful of foods: tree nuts such as pistachios, walnuts, or cashews; cow’s milk; eggs; wheat or wheat flour; peanuts; fish; shellfish; and soy. In one analysis, peanuts and tree nuts accounted for 90% of 32 cases of food-induced anaphylaxis.22 The list of foods reported to be associated with anaphylaxis is listed in Table 2. Specific foods are discussed below, with particular emphasis on peanut allergy.
Peanuts. Peanut allergy represents the most significant food allergy in the United States in terms of morbidity and mortality.54
Peanut allergy generally develops at an early age and often is a lifelong disorder. In one large registry of 4685 patients with peanut allergy, the first reaction to peanuts occurred at a median age of 14 months.55
Evaluation for peanut allergy includes a careful history taking, skin-prick tests, radio-allergosorbent tests (RAST), and, perhaps, a supervised oral food challenge. Patients who have had unequivocal symptoms of allergy after the isolated ingestion of a peanut product with evidence of peanut-specific allergies by IgE testing, either by RAST or positive skin-prick test, do not need to undergo oral peanut challenges to establish the diagnosis.56 Patients with peanut-specific serum IgE levels of at least 15 kU per liter have a likelihood of an allergic reaction of 95% or greater if they ingest peanuts. If serum IgE levels are less than 15 kU per liter with no clear-cut history of peanut-induced symptoms, a physician-supervised food challenge may be necessary to make the definitive diagnosis. A double-blind placebo-controlled challenge is considered the gold standard in diagnosing food allergy and should be conducted in a hospital setting.56
The prevalence of peanut allergy in the United States nearly doubled in the 10-year interval from 1980-1984 until 1990-1994. A national survey indicated that approximately 3 million Americans are allergic to peanuts, tree nuts, or both.57-59 A survey from 1988-1994 indicated that approximately 6% of Americans had serologic evidence for sensitivity to peanuts, based upon the presence of IgE antibodies specific to peanut proteins,60 and the prevalence of peanut allergy in the general population has been cited as 1.5% in a recent report.61 Peanut allergy seems to be a Western phenomenon. Although peanut allergy is nearly unknown in China, Chinese families who emigrate to the United States are observed to have the same prevalence of peanut allergy as other American children. The United States, as opposed to China, tends to roast its peanuts. Also unlike China, more than 40% of the U.S. peanut crop is consumed as peanut butter.
Although most patients with peanut allergy know to avoid peanuts, unanticipated exposures, as in the inhalation of peanut dust in airplanes, may result in an allergic reaction. Highly processed oils, such as acid-extracted and heat-distilled oils, do not contain peanut protein and may be consumed safely.62 On the other hand, cold-processed or extruded peanut oils contain peanut protein and may induce allergic reactions. Cross-contamination may occur in restaurants, as when the same pan is used to cook foods containing peanuts and foods without peanuts. Because of the ubiquitous nature of peanuts and peanut products, accidental exposures occur frequently despite patient attempts to avoid peanut-containing foods. The danger of casual exposure to peanut products has not been resolved. One report in 2003 noted that, of 30 children with documented peanut allergy, none had any respiratory or systemic symptoms from the inhalation of peanut butter held 30 cm away, or to 0.2 mL of peanut butter applied to intact skin for one minute.63 Yet, anecdotal reports of allergic reactions from the mere presence of peanut butter in a room, kissing someone who has eaten the offending food, or inhaling vapors from cooking food mean that the dose needed to provoke an allergic reaction varies among individuals.64 Table 7 summarizes features of the management of peanut allergy.59
Table 7.
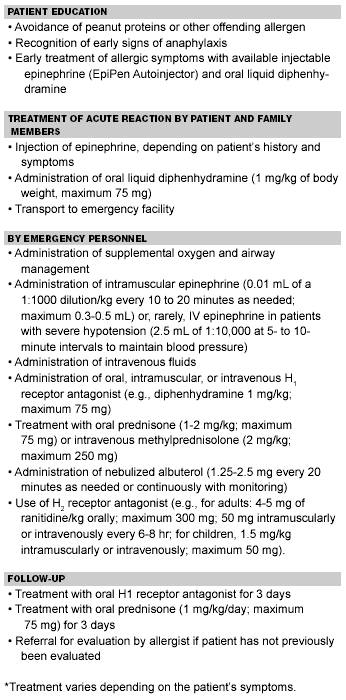
General Treatment of Food Allergic Reactions4,19
Tree Nuts. Tree nuts most commonly responsible for allergic reactions include walnuts, cashews, almonds, and hazelnuts, in that order. Less frequently, pecans, chestnuts, Brazil nuts, pine nuts, macadamia nuts or pistachios, coconuts, or acorns are responsible for allergic reactions. Specific allergens to individual tree nuts have been well-characterized.65 Tree nuts and peanuts accounted for 94% of food-induced anaphylaxis deaths in one report.41 In another national registry, all of the fatal allergic reactions to foods for individuals older than 6 years reported were caused by either peanuts or tree nuts.47
Milk. Milk allergy nearly always presents in the first year of life, soon after introduction of cow’s milk or cow’s milk-based infant formula. Reported prevalences of cow milk allergy (CMA) range from 0.5-7.5% of children.66 Adult CMA is rare and generally milder than in infants.67 Most infants develop gastrointestinal symptoms, although 50-70% have cutaneous manifestations, and 20-30% have respiratory symptoms.68 Milk allergy affects 2-3% of infants. Of 39 infants studied in one report, 21 had positive skin or serum IgE tests to milk, and 18 had non-IgE milk intolerance. Of the total group, 56% could tolerate milk by 1 year of age, and 87% could tolerate milk by age 3.69 Casein and beta-lactoglobulin are the most allergenic proteins in cow’s milk. CMA may be IgE-mediated or non-IgE-mediated. (See Table 8.) Non-IgE-mediated reactions in CMA generally entail Type III (Arthus type), inducing vasculitis, or Type IV (delayed), mediated by sensitized T lymphocytes.
Table 8.
Manifestations of Cow Milk Allergy66-68
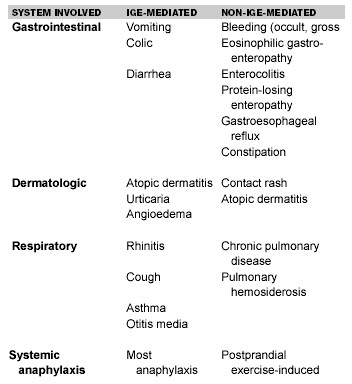
In Type I reactions, total IgE levels typically are elevated. Patients may present with colic, diarrhea, or vomiting. Cutaneous manifestations may include atopic dermatitis, urticaria, angioedema, or frank anaphylaxis. Non-IgE-mediated manifestations include pulmonary hemosiderosis, chronic pulmonary disease, enterocolitis, protein-losing enteropathy, gastroesophageal reflux, and possibly fecal blood, leukocytes, or eosinophils.67 Chronic constipation and anal fissures occur more frequently in patients with CMA.70 Treatment is based on avoidance of cow’s milk as strictly as possible. Soybean hydrolysate formulas, or hypoallergenic formula from hydrolyzed casein or whey tend to be well-tolerated. Allergic reactions to even extensively hydrolyzed formulas do occur rarely.71,72 CMA tends to be temporary, with tolerance reported in approximately one-half of children by age 3-473 and about 80% by their fifth birthday.4
In the management of milk allergy, infants may be fed breast milk or hydrolyzed cow’s milk-based formula. Resolution of symptoms with a soy formula, or whey-based and casein-based formulas (i.e., Nutramigen, Alimentum) is highly suggestive of CMA. Hydrolysis reduces the allergenicity of milk proteins. Feeding partially hydrolyzed formula—or breast feeding exclusively for four months—has been stated to decrease the overall incidence of atopic disease.74
Soy. Soy has been listed by the Food and Agriculture Organization of the United Nations as one of the eight most significant food allergens. At least 16 potential soy protein allergens have been identified. Conversely, soy has a long history of successful use in managing milk allergies in infants.75 Symptoms of soy allergy may include typical IgE-mediated features, as well as non-IgE-mediated ones: hematochezia, malabsorption, and other gastrointestinal symptoms. The prevalence of soy allergy is a function of when it is introduced into regional diets, but appears to affect 1-6% of infants.76,77 Most children can tolerate soy products by school age.
Egg. The prevalence of egg allergy in the pediatric population has been estimated at 1.6-2.6%, but is higher in the population with atopic illness.78,79 Approximately 85% of egg-allergic children have cutaneous symptoms, while 60% have gastrointestinal symptoms. In one report, 44% of egg-allergic children were able to tolerate egg products in their diet by school age, but egg allergy persisted in the remaining 56%.80 Dietary protein enterocolitis, manifested by recurrent vomiting or diarrhea, typically resolves by 18-24 months of age. Elimination of the offending antigen generally will result in resolution of symptoms within 72 hours.81
Wheat Allergy. Ingestion of wheat products and inhalation of wheat flour are the two major routes of sensitization. Wheat allergy may manifest as occupational (baker’s) asthma, T-cell mediated intestinal inflammation, urticaria, angioedema, bronchial obstruction, or dermatitis herpetiformis. Wheat, along with milk, eggs, soy, peanuts, and fish, account for 90% of food-allergic reactions in children.82 While wheat typically may not be introduced into an infant’s diet until approximately 5 months of age, sensitization to wheat may occur through maternal milk during exclusive breastfeeding.83
Celiac disease and protein enteropathy are clinical manifestations of delayed cell-mediated non-IgE pathology. In wheat allergy, ingested wheat results in enteropathy, atopic dermatitis, vomiting, and possibly exercise-induced anaphylaxis. Presentation of celiac disease may include malabsorption, diarrhea, growth impairment, associated autoimmune disorders, and lymphoma with continued gluten exposure.84 It is associated with sensitivity to omega-5gliadin found in wheat, rye, and barley. In the United States, more than 2 million people are affected.85 Wheat allergy is generally transient and T cell mediated.86 Celiac disease is associated with HLA-DQ2 in over 90% of cases.87 RAST testing and skin-prick tests seems to be less accurate than IgE enzyme-linked immunosorbent assay (ELISA) with omega-5 gliadin.82
Shellfish. It appears that most adults with shellfish allergy remain allergic their entire lives.46 There are, however, cases of individuals becoming tolerant to shrimp.88
Other Foods. The list of other foods that may induce allergy is extensive: Sesame, poppy, and mustard seed allergies, while less common, potentially persist indefinitely.46 Kiwi fruit allergy has been described.89 Cross-reactivity occurs as well. For example, latex-sensitive patients may be allergic to avocado, banana, chestnut, fig, papaya, and kiwi.90,34
Treatment
As with all emergencies, airway management takes priority over all else initially. Oxygen should be administered. First aid treatment for anaphylactic reactions to food generally starts with epinephrine, typically injected intra-muscularly into the vastus lateralis muscle of the thigh. Oral H1-antihistamines such as diphenhydramine are used in milder cases.91
Management of Anaphylactic and Allergic Reactions
Patients who have an anaphylactic reaction should be treated with intramuscular epinephrine. The dose to be administered is 0.01 mg/kg intramuscularly (IM) in children. The IM route injected into the thigh has been shown to produce peak-plasma epinephrine levels higher than injection into the upper arm or subcutaneously.92 Intravenous epinephrine has proven fatal in non-life-threatening situations.93
Histamine H1 and H2 receptor antagonists may be given intravenously, intramuscularly, or orally. The H1 antihistamine diphenhydramine typically is administered 1 mg/kg up to 75 mg per dose and a maximum of 400 mg/day. The H2 antihistamine ranitidine may be given at a dose of 4 mg/kg up to 300 mg. Inhaled albuterol, oxygen, and systemic corticosteroids should be given.41 The role of corticosteroids in the management of anaphylaxis is unclear. Typical doses include 1 mg/kg of prednisone or 1-2 mg/kg intravenously of methylprednisolone.
More than 90% of biphasic responses occur within four hours of the initial reaction, making it important to observe patients for four hours prior to discharge from the emergency department. An association has been reported between delay in administration of epinephrine and an increase in biphasic anaphylactic reactions.94
The use of activated charcoal to prevent further absorption of food from the gut has been proposed,95 but never shown to be efficacious.19 At discharge, a course of steroids, such as 1 mg/kg of oral prednisone for several days seems reasonable, although this has not been demonstrated to prevent a biphasic response. Systemic steroids generally are effective in treating chronic IgE-mediated disorders such as atopy and asthma, as well as non-IgE-mediated disorders such as allergic eosinophilic esophagitis and gastroenteritis.4 An oral antihistamine often is recommended, although this has not been shown to prevent recurrent symptoms.
Specific therapies for peanut allergies appear promising. Monthly injections of humanized recombinant anti-IgE antibodies may reduce the levels of IgE bound to mast cells and basophils enough to suppress allergic activation to small amounts of peanut protein.
Another area of research entails using recombinant peanut proteins that have substitutions of critical amino acids within the IgE-binding epitopes to prevent the activation of IgE-mediated reactions. Engineered recombinant proteins have been shown to reverse sensitivity to peanuts on a model of peanut-induced anaphylaxis in mice without inducing IgE-mediated anaphylaxis.56
Prevention and Patient Education
Prevention is a cornerstone of therapy, especially regarding peanut exposure, the most common cause for anaphylactic death. Even in the non-IgE-mediated food hypersensitivities, allergen avoidance is the mainstay of therapy. Patients with peanut allergy must learn to avoid the accidental ingestion of peanuts. It is advisable to avoid high-risk situations such as buffets. They should check food labels for the presence of peanuts and avoid high-risk situations such as foods served at buffets and unlabeled candies and desserts. Many restaurants use the same pan to cook foods containing peanuts and foods without peanuts. Unanticipated exposures typically occur every 3-5 years in peanut-allergic patients, such as inhalation of peanut dust in airplanes.96
Patients with a history of anaphylactic reactions to foods should have injectable epinephrine and liquid diphenhydramine available for use in case of accidental ingestion of an allergen. Schools may be unprepared for food-allergic children; it is advisable that injectable epinephrine (Epi-Pen) be available at the school if a child’s allergy is known to be IgE-mediated. The dosage recommended is 0.01 mg/kg of a 1:1000 solution administered intramuscularly. Since the EpiPen Jr contains 0.15 mg of epinephrine, and the adult EpiPen contains 0.30 mg, the dose may have to be extrapolated for children weighing between 15 and 30 kg. An upper cutoff of 20 kg has been proposed for EpiPen Jr dosing. There should be at least one person on the school’s premises trained in its use. Many schools also have created peanut-free tables in their cafeterias.41
It is questionable as to whether allergy to foods such as peanuts is preventable. However, it is recommended by the Department of Health in the United Kingdom that mothers in families at high risk, such as those with a history of atopy, avoid eating peanuts during lactation and that they avoid giving their infants peanut products for the first three years of life.97 It has been proposed that mandatory food-labeling laws and manufacturing practices be enacted to prevent the inadvertent ingestion of contaminated products.98 It is recommended that the mother eliminate the same foods to which the child is allergic, during breast-feeding.99 Educational materials are available online through the Food Allergy and Anaphylaxis Network at www.foodallergy.org to assist patients with avoiding known allergens and in coping with their allergies.
It has been proposed that delayed entry of specific foods into the diet may prevent the development of food allergies. Although no studies has confirmed the efficacy of such practice, the following have been proposed as a general recommendation: no peanuts until age 4, no egg until age 18 months, and no cow’s milk until 12 months.100 The American Academy of Pediatrics recommends that major allergens such as peanuts, nuts, and seafood not be introduced until after 3 years of age for high-risk infants.
Medical information concerning their condition should be present at all times for patients at risk for food-induced anaphylaxis (i.e., such as a medical identification bracelet).
Implications for Industry
Food processing equipment was not designed historically for removal of allergen residues. The food industry has had to modify the way it manufactures foods in many cases to address allergen control. This may be performed, for example, by manufacturing all dairy-containing foods together, or all egg-containing foods together, and so on. When shared equipment is used, effective clean-up may prove unwieldy. Many companies have gone so far as to use color-coding for sanitation, with each color dedicated to a type of allergen. The question has not been answered as to whether vacuuming or hand-wiping is enough to ensure that foods remain sufficiently allergen-free.101
Detection of allergenic residues in foods has improved with the advent of enzyme-linked immunosorbent assays (ELISA) for allergenic foods. Commercial ELISAs are available for detection of peanuts, whey, sesame seeds, and wheat gluten. In 1999, a total of 68 recall actions involving 236 food products were made by the U.S. Food and Drug Administration for undeclared antigens.102 The major line of defense that a food-allergic consumer has against a reaction is the ingredient listing on packaged food products. Not surprisingly, many severe food-allergic reactions occur in food-service settings.
Prognosis
It appears that approximately 20% of young infants who have allergic reactions to peanuts will outgrow their allergy, especially if they have low levels (< 5 kU/liter) of peanut-specific serum IgE antibodies in infancy.103 This may be evidenced by conversion of the skin-prick test from positive to negative. However, patients who have outgrown their peanut allergy may have persistently positive skin-prick tests for many years.19
Allergic reactions to milk, eggs, soy, and wheat generally are outgrown by age 3-5 years, whereas sensitivity to peanuts, tree nuts, seeds, fish, and shellfish often persist.103
Food Allergy Mimics
Adverse food reactions include any abnormal reaction resulting from the ingestion of a food and might constitute a food intolerance. For example, scombroid fish poisoning represents a histamine reaction to ingested food, but is not an immune-mediated reaction. A patient may experience an exaggerated pharmacologic response to natural or added chemical agents such as tyramine or other vasoactive amines in wines, as another example.
Vasodepressor syncope following an injection should not induce pruritus or respiratory difficulty. Pulmonary embolism, seizure disorder, and possible aspiration all may present with sudden dyspnea or depressed consciousness.
Allergy denotes an immunologically mediated reaction. Milk intolerance may not be immune mediated. Cow milk intolerance is caused mostly by inadequate digestion, either of the milk sugar lactose or occasionally of the fat in the milk. Adverse reactions to foods may stem from enzyme deficiencies such as lactose intolerance, but do not fall under the category of food allergy.67 Lactase enzyme in the brush border of the small intestine hydrolyzes lactose into the readily absorbed glucose and galactose. After acute gastroenteritis, for example, lactase deficiency may take days to weeks to normalize. Lactose that passes un-hydrolyzed to the colon holds water, becomes fermented by colonic flora, and produces hydrogen and carbon dioxide. Resultant bloating, flatulence, abdominal pain, or watery diarrhea constitutes lactose intolerance, not milk allergy. Lactose intolerance affects primarily adults, produces gastrointestinal symptoms only, and is treatable with reduction of milk intake or with lactase replacement—all in contrast to CMA.67
Irritable bowel syndrome patients may have an increased number of mast cells in the ileocecal region, especially in those patients with diarrhea predominating as a symptom. An immunologically based reaction to food has been suggested as a cause. No skin prick test or IgE antibodies have ever been demonstrated to identify an offending food antigen.104
Unproven Therapies and Diagnostic Tests
Alternative and complementary medicine approaches to allergic disorders commonly are employed by patients. It is worth noting some of these, especially as the term food allergy may be used by the public to describe a multitude of symptoms and ailments presumably related to food ingestion. Physicians use the term to describe an immune response to a food that results in an adverse reaction. Because of the popularity of the topic with the public, some treatments are worthy of mention, even if their worth in the management of the food-allergic patient is unproven.
Food specific IgG panels have been advertised as IgG radio-allergosorbent tests. Its proponents state that such antibodies can identify food intolerances that cause or contribute to chronic fatigue, headache, irritable bowel syndrome, arthritis, or difficulty concentrating.105 One recent study recently showed no difference in specific IgG antibody values between milk-allergic patients and age-matched controls.106 At present there is no evidence to support the diagnostic use of food-specific IgG in any particular disorder.
Provocation-neutralization testing to foods involves sublingual or intradermal provocation by a test antigen, followed by an observation for wheal response after 10 minutes. A positive challenge is considered if the patient develops drowsiness, dry mouth inability to concentrate, or headache. At least one Canadian group concluded that provocation of symptoms by intradermal testing should not be used to make diagnostic of therapeutic decisions.107
Applied kinesiology entails muscle response testing. Practitioners state that they can detect both IgE- and non-IgE-mediated allergy or intolerance based on negative energy balances manifesting as muscle weakness. The patient holds a vial with a specific food item in one hand while the practitioner tests the muscle strength of the opposite arm. One study concluded that kinesiology as a diagnostic tool was no better than random guessing.108
Chinese herbal therapies have been examined for the prevention of peanut-induced anaphylaxis in a murine model. The treated mice had a decrease in peanut-specific IgE compared to the treated group and, on challenge, exhibited no allergic symptoms compared to placebo. The active components of the mixture have not been identified.109
Cytotoxicity and in-vitro cell assays attempt to find changes in cell morphology when a fresh drop of blood is added to a dried film of food extract. Any morphological change on blood mononuclear cells was considered positive. Pulse test entails measuring a change in pulse after a sublingual drop, intradermal injection, or open challenge is applied. A change in pulse of 16 beats per minute from baseline is considered positive. There are no blinded clinical trials to support use of the above tests.105
Future Therapies and Diagnostics
Peanuts are responsible for the greatest number of deaths due to food allergy, and represent the source for promising research. The three major peanut allergens are Ara h1, Ara h2, and Ara h3. Prevention of IgE binding to these three antigens is the basis of the experimental hypoallergenic peanut. Single amino acid changes have diminished IgE binding and may abrogate the allergic response.110,111 By using a mouse model of peanut anaphylaxis, heat-killed E. coli containing mutated recombinant Ara allergens were injected or administered rectally to mice. The mice given engineered hypoallergenic peanut allergens produced less severe reactions and lower peanut-specific IgE levels compared to sham-treated mice.112
Wheat allergy research also has shown some preliminary results that may have clinical application. Glutenins and gliadins collectively are referred to as glutens or prolamins, and are specific seed storage proteins capable of eliciting an immune response. Site-directed mutagenesis recently has been shown to abrogate the T cell stimulatory response by substitution of a prolene in glutenin and gliadin proteins. The T cell response was not completely abolished, suggesting that other amino acid substitutions might be needed before the T cell response might be completely abolished.113
Immunotherapy by oral route or systemic injections shows some promising preliminary results. In one trial of six patients with peanut allergy, maintenance was reached in four patients, who could then tolerate more peanut.114
Promising results have been published in a recent trial using anti-IgE antibodies.2,115 Anti-IgE complexes with unbound or free IgE, preventing its binding to effector cells to inhibit the allergic reaction. The use of humanized, recombinant anti-IgE therapy in peanut allergic individuals significantly can increase the quantity of peanut necessary to induce an allergic response relative to controls. The threshold of sensitivity was noted to increase from a level of approximately half a peanut to approximately 9 peanuts.115 The use of prophylactic anti-IgE may show promise in preventing severe IgE-mediated food allergic reactions, although it would have to be administered indefinitely.
Probiotics are cultures of potentially beneficial bacteria that positively affect hosts by restoring normal intestinal permeability and gut microbiology.116 The intent of probiotic administration is a beneficial change in the intestinal microflora, with improvement in the nonimmune and immune resistance in the intestinal tract. The definition of probiotics is evolving, since the term includes normal live micro-organisms as well as genetically modified strains and biotechnology derived products.117 Most used are bifidobacteria and lactobacilli, the latter in particular used for yogurt production. For example, L. acidophilus and L. rhamnosus have accelerated recovery from food allergy symptoms, including infants with eczema and cow’s milk allergy.118
The development of allergen chips has been proposed. Micro-matrices containing approximately one hundred allergens deposited as spots on a glass slide would enable detection of specific IgEs with only 0.04 mL of serum.119,120
Applying DNA technology, up to 40 food allergens have been produced in recombinant form, implying standardized quality and unlimited quantity of the respective proteins being investigated. Experiments with recombinant food allergens appear promising.53
Finally, recent advances in technology have enabled investigators to map epitopes of specific food allergens and to study reactions to specific amino acid sequences (sequential epitopes) vs. to the tertiary structure of the protein (conformational epitopes). This is important in that heating or hydrolysis may alter the tertiary structure of a protein, altering the conformational epitope so that a person can tolerate its ingestion. If the patient’s IgE is directed at sequential epitopes, on the other hand, that patient is less likely to ever develop tolerance.121,122
Conclusions
The prevalence of food allergy appears to be rising. Food-induced anaphylaxis is the leading cause of anaphylaxis in the USA. The management of food allergies continues to consist of educating patients on how to avoid relevant allergens, to recognize early symptoms of an accidental ingestion, and to initiate emergency treatment when indicated. Strict dietary vigilance and the ability to self-treat an anaphylactic reaction are critical components of the management of food allergy. A fraction of individuals with allergy to foods traditionally believed to persist indefinitely have demonstrated loss of allergy and clinical tolerance. A number of immunomodulary therapies show promise for effective therapy. Until reliable preventive treatment is available, prompt treatment of anaphylaxis with epinephrine remains the most important clinical response to food-induced anaphylaxis.
References
1. Sampson HA. Food allergy. Part 1. Immunopathogenesis and clinical disorders. J Allergy Clin Immunol 1999;103:717-728.
2. Eigenmann PA. Future therapeutic options in food allergy. Allergy 2003;58:1217-1223.
3. Niestijl J, Kardinall A, Huijbers G, et al. Prevalence of food allergy and intolerance in the adult Dutch population. J Allergy Clin Immunol 1994;93:446-456.
4. Sampson HA. Update on food allergy. J All Clin Immunol 2004;113:805-819.
5. Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmstead County: A population-based study. J Allergy Clin Immunol 1999;104:452-456.
6. Sloan A, Powers M. A perspective on popular perceptions of adverse reactions to foods. J Allergy Clin Immunol 1986;78: 127-133.
7. Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep 2004;4:43-46.
8. Yunginger JW, Sweeney KG, Sturner WQ, et al. Fatal food-induced anaphylaxis. J Am Med Assoc 1988:260:1450-1452.
9. Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 2001;107:191-193.
10. Sampson HA. Fatal food-induced anaphylaxis. Allergy 1998;53:Suppl:125-130.
11. Hill DJ, Hosking CS, Heine RG. Clinical spectrum of food allergy in children in Australia and South-east Asia: Identification and targets for treatment. Ann Med 1999;31:272-281.
12. Halken S. Prevention of allergic disease in childhood: Clinical and epidemiological aspects of primary and secondary allergy prevention. Ped All and Immunol 2004;15:Suppl 16:4-5, 9-32.
13. Fogg MI, Spergel JM. Management of food allergies. Expert Opinion on Pharmacology 2003;4:1025-1037.
14. Mayer L. Mucosal immunity. Pediatr 2003;11:1595-1600.
15. Vadas P, Wai Y, Burks W, et al. Detection of peanut allergens in breast milk of lactating women. JAMA 2001;285: 1746-1748.
16. Hatahet R, Kirch F, Kanny G, et al. Sensibilization aux allergenes d’arachide chez lez nourrissons de moins de quatre mois: A propos de 125 observation. Rev Fr Allergol Immunol Clin 1994;34:377-381.
17. Zeiger RS, Heller S, Mellon MH, et al. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: A randomized study. J Allergy Clin Immunol 1989;84:72-89.
18. Beyer K, Morrow E, Li XM, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol 2001; 107:1077-1081.
19. Sampson HA. Peanut allergy. N Engl J Med 2002;346:1294-1299.
20. Warner J, Miles E, Jones A, et al. Is deficiency of interferon gamma production by allergen triggered cord blood cells a predictor of atopic eczema? Clin Exp Allergy 1994;24: 423-430.
21. Frank L, Marian A, Visser M, et al. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatr Allergy Immunol 1999;10:27-32.
22. Sampson HA, Mendelson LM, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 1992;327:380-384.
23. Varjonen E, Vainio E, Kalimo K. Life-threatening, recurrent anaphylaxis caused by allergy to gliadin and exercise. Clin Exp Allergy 1997;27:162-166.
24. Shimamoto SR, Bock SA. Update on the clinical features of food-induced anaphylaxis. Curr Opin Allergy Clin Immunol 2002;2:211-216.
25. Rothenberg ME, Mishra A, Collins MH, et al. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 2001;108:891-894.
26. Orenstein SR, Shalaby TM, DiLorenzo C, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: A clinical series of 30 children. Am J Gastroenterol 2000;95:1422-1430.
27. Liacouras CA, Markowitz JE. Eosinophilic esophagitis: A subset of eosinophilic gastroenteritis. Curr Gastroenterol Rep 1999;1:253-258.
28. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004;113:11-28.
29. Lee SK, Kniker WT, Cook CD, et al. Cow’s milk-induced pulmonary disease in children. Adv Pediatr 1978;25:39-57.
30. Greaves MW. Chronic urticaria. J Allergy Clin Immunol 2000;105:910-922.
31. Nicolas ME, Krause PK, Gibson LE, et al. Dermatitis herpetiformis. Int J Dermatol 2003;42:588-600.
32. Burks AW, Jones SM, Wheeler JG, et al. Food allergy: Current knowledge and future directions. Immunol Allergy Clin North Am 1999;19:533-554.
33. Lin RY, Schwartz LB, Curry A, et al. Histamina and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J Allergy Clin Immunol 2000;106: 65-71.
34. Bahna SL. Diagnosis of food allergy. Ann Allergy, Asthma and Immunol 2003;90 (Suppl3):77-80.
35. Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol 1998;9:186-191.
36. Rosen J, Selcow J, Mendelson L, et al. Skin testing with natural foods in patients suspected of having food allergiesis it necessary? J Allergy Clin Immunol 1994;93:1068-1070.
37. Lavaud F, Sabouroud D, et al. Cross reactions involving natural rubber latex. Clin Rev Allergy Immunol 1997;15:429-447.
38. Moneret-Vautrin DA, Kanny G, Fremont S. Laboratory tests for diagnosis of food allergy: Advantages, disadvantages and future perspectives. Allergie at Immunologie 2003;35: 113-119.
39. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 1997;100: 444-451.
40. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001;107:891-896.
41. Fogg MI, Pawlowski NA. Anaphylaxis. Pediatric Case Reviews 2003;3:75-82.
42. Garcia AM, Boyano MT, Diaz Pena JM, et al. Specific IgE levels in the diagnosis of immediate hypersensitivity to cow’smilk protein in the infant. J Allergy Clin Immunol 2001;107:185-190.
43. Boyano MT, Garcia-Ara C, Diaz-Pena JM, et al. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy 2001;31:1464-1469.
44. Rance F, Abbal M, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol 2002;109: 1027-1033.
45. Clark AT, Ewan P. Interpretation of tests for nut allergy in one thousand patients, in relation to allergy or tolerance. Clin Exp Allergy 2003;33:1041-1045.
46. Kagan RS. Food allergy: An overview. Environmental Health Perspectives 2003;111:223-225.
47. Bock S, Sampson H, Atkins F, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J Allergy Clin Immunol 1988;82:986-987.
48. Huijbers GB, Colen AA, Lansen JJ, et al. Masking foods for food challenge: Practical aspects of masking foods for a double blind, placebo-controlled food challenge. J Am Diet Assoc 1994;94:645-649.
49. Noe D, Bartemucci L, Mariani N, et al. Practical aspects of preparation of foods for a double-blind placebo-controlled food challenge. Allergy 1998:53 (Suppl):75-77.
50. Majamaa H, Moisio P, Holm K, et al. Cow’s milk allergy: Diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy 1999;54:346-351.
51. Majamaa H, Moisio P, Holm K, et al. Wheat allergy: Diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy 1999;54:851-856.
52. Niggemann B. Evolving role of the atopy patch test in the diagnosis of food allergy. Curr Opinion Allergy Clin Immunol 2002;2:253-256.
53. Bohle B, Vieths S. Improving diagnostic tests for food allergy with recombinant allergens. Methods (Duluth) 2004;32: 292-299.
54. Bock SA, Atkins FM. The natural history of peanut allergy. J Allergy Clin Immunol 1989;82:986-997.
55. Sicherer SH, Furlong TJ, Munoz-Furlong A, et al. A voluntary registry for peanut and tree nut allergy: Characteristics of the first 5149 registrants. J Allergy Clin Immunol 2001;108: 128-132.
56. Sampson HA. Food-induced anaphylaxis. Novartis Foundation Symposium 2004;257:161-171.
57. Sicherer SH, Munoz-Furlong A, Burks AW, et al. Prevalence of peanut and tree nut allergy in the U.S. determined by a random digit dial telephone survey. J Allergy Clin Immunol 2001;107:191-193.
58. Sampson HA. Immunological approaches to the treatment of food allergy. Pediatr Allergy Immunol 2001:12 Suppl 14: 91-96.
59. Sampson HA. Managing peanut allergy. BMJ 1996;312: 1050-1051.
60. Chiu L, Sampson HA, Sicherer SH. Estimation of the sensitization rate to peanut by prick skin test in the general population: Results from the National Health and Nutrition Examination Survey 1988-1994. J Allergy Clin Immunol 2001;107; Suppl: S192. Abstr.
61. Grundy J, Matthews S, Bateman B, et al. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol 2002;110:784-789.
62. Hourihanc JOB, Bedwani SJ, Dean TP, et al. Randomized, double-blind, cross-over challenge study of allergenicity of peanut oils in subjects allergic to peanuts. BMJ 1997;314: 1084-1088.
63. Simonte S. Relevance of casual contact with peanut butter in children with peanut allergy. J Allergy Clin Immunol 2003; 112:183-189.
64. Taylor SL, Hefle SL, Bindslev-Jensen C, et al. Factors affecting the determination of threshold doses for allergenic foods: How much is too much? J Allergy Clin Immunol 2002;109: 24-30.
65. Roux KH, Teuber SS, Sathe SK. Tree nut allergens. Int Arch Allergy Immunol 2003;131:234-244.
66. Host A, Bahna SL. Cow’s milk allergy. In: Frieri M, Kettlehut B, editors. Food Hypersensitivity and Adverse Reactions: A Practical Guide for Diagnosis and Management. New York. Marcel Dekker 1999: 99-111.
67. Bahna SL. Cow’s milk allergy versus cow milk intolerance. Ann Allergy Asthma Immunol 2002;89 (suppl 1):56-60.
68. Host A. Cow’s milk protein allergy and intolerance in infancy. Some clinical, epidemiological, and immunological aspects. Pediatr Allergy Immunol 1994;5:5-36.
69. Host A, Halken S. A prospective study of cow’s milk allergy in Danish infants during the first 3 years of life: Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990;45:587-596.
70. Magazzu G, Scoglio R. Gastrointestinal manifestations of cow’s milk allergy. Ann Allergy Asthma Immunol 2002;89 (6 Suppl I):65-68.
71. Saylor JD, Bahna SL. Anaphylaxis to casein hydrosylate formula. J Pediatr 1991;118:71-74.
72. Ragno V, Giampetro P, Bruno G, et al. Allergenicity of milk protein hydrolysate formulae in children with cow’s milk allergy. Eur J Pediatr 1993;152:760-762.
73. Bishop W, Hill D, Hosking C. Natural history of cow milk allergy: Clinical outcome. J Pediatr 1990;116:862-867.
74. Chandra RK. Food hypersensitivity and allergic diseases. Eur J Clin Nutr 2002;56 Suppl 3:S 54-56.
75. Cordle CT. Soy protein allergy: Incidence and relative severity. J Nutr 2004;134:1213S-1219S.
76. Giampetro B, Buercio M, Giovaaini L, et al. Soy allergy is not common in atopic children: A multicenter study. Pediatr Allergy Immunol 1997;8:190-193.
77. Magnolfi CF, Zani G, Lecava L, et al. Soy allergy in atopic children. Ann Allergy Asthma Immunol 1996;77:197-201.
78. Danneus A, Jhansson S, Foucard T, et al. Clinical and immunological aspects of food allergy in childhood: Estimation of IgG, IgA, and IgE antibodies to food antigens in children with food allergy and atopic dermatitis. Acta Paediatr Scand 1977;66:31-37.
79. Eggesbo M, Botten G, Halvorsen R, et al. The prevalence of allergy to egg: a population-based study in young children. Allergy 2001;56:403-411.
80. Ford R, Taylor B. Natural history of egg hypersensitivity. Arch Dis Child 1982;57:649-652.
81. Burks W. Current understanding of food allergy. Ann NY Acad Sci 2002;964:1-12.
82. Palosuo K. Update on wheat hypersensitivity. Curr Opin Allerg & Clin Immunol 2003;3:185-188.
83. Linna O. Specific IgE antibodies to undigested cereals. Allergy 1996;51:849-850.
84. Williamson D, Marsh MN. Celiac Disease. Mol Biotechnol 2002;22:293-299.
85. Harder B. Target: Celiac disease. Therapies aimed to complement or replace the gluten-free diet. Science News 2003;163: 392-393.
86. Perr HA. Novel foods to treat food allergy and gastrointestinal infection. Current Gastroenterology Reports 2004;6: 254-260.
87. Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: Genetic mapping and role in pathogenesis. Gastroenterology 1993;105:910-922.
88. Daul C, Morgan J, Lehrer S. The natural history of shrimp hypersensitivity. J Allergy Clin Immunol 1990;86:488-493.
89. Lucas JS, Lewis SA, Hourihane JO. Kiwi fruit allergy: A review. Ped All and Immunol 2003;14:420-428.
90. Blanco C, Carrillo T, Castillo R, et al. Latex allergy: Clinical features and cross-reactivity with fruits. Ann Allergy 1994;73: 309-314.
91. Simons FE. First-aid treatment of anaphylaxis to food: Focus on epinephrine. J Allergy Clin Immunol 2004;113:837-844.
92. Simons FE, GU X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001;108:871-873.
93. Johnston SL, Unsworth J, Gompels MM. Lesson of the week: Adrenaline given outside the context of life threatening allergic reactions. Br Med J 2003;326:589-590.
94. Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics 2000;106:762-766.
95. Vadas P, Perelman B. Activated charcoal forms non-IgE binding complexes with peanut proteins. J Allergy Clin Immunol 2003;112:175-179.
96. Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 1998;102:131. Abstract.
97. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Peanut Allergy. London: Department of Health 1998: 1-57.
98. Altschul AS, Sicherer DL, Munoz-Furlong A, et al. Manufacturing and labeling issues for commercial products: Relevance to food allergy. J Allergy Clin Immunol 2001;108:468.
99. Collier S, Fulhan J, Duggan C. Nutrition for the pediatric office: Update on vitamins, infant feeding and food allergies. Curr Opin Pediatr 2004;16:14-20.
100. Sicherer SH. The impact of maternal diets during breastfeeding on the prevention of food allergy. Curr Opin Allergy Clin Immunol 2002;2:207-210.
101. Hefle SL, Taylor SL. Food allergy and the food industry. Curr Allerg and Asthma Reports 2004;4:55-59.
102. Vierk K, Falci K, Wolyniak C, et al. Recalls of foods containing undeclared allergens reported to the US Food and Drug Administration, fiscal year 1999. J Allergy Clin Immunol 2002;109:1022-1026.
103. Skolnick HS, Conover-Walker MK, Koerner CB, et al. The natural history of peanut allergy. J Allergy Clin Immunol 2001;107:367-374.
104. Zar S, Kumar D, Kumar D. Role of food hypersensitivity in irritable bowel syndrome. Minerva Medica 2002;93:403-412.
105. Teuber SS, Porch-Curren C. Unproved diagnostic and therapeutic approaches to food allergy and intolerance. Curr Opin Allergy Clin Immunol 2003;3:217-221.
106. Szabo I, Eigenmann PA. Allergenicity of major cow’s milk and peanut proteins determined by IgE and IgG immunoblotting. Allergy 2000;55:42-49.
107. Fox RA, Sabo BMT, Williams TPW, et al. Intradermal testing for food and chemical sensitivities: A double-blind controlled study. J Allergy Clin Immunol 1999;103:907-911.
108. Ludtke R, Kunz B, Seeber N, et al. Test reliability and validity of the kinesiology muscle test. Complement Ther Med 2001;9:141-145.
109. Li XM, Kleiner G, Huang CK, et al. Murine model of atopic dermatitis associated with food hypersensitivity. J Allergy Clin Immunol 2001;107:693-702.
110. Bannon GA, Shin D, Maleki S, et al. Tertiary structure and biophysical properties of a major peanut allergen, implications for the production of a hypoallergenic protein. Int Arch Allergy Immunol 1999;118:315-316.
111. Rabjohn P, West CM, Connaughton C, et al. Modification of peanut allergen Ara h3: Effects on IgE binding and T-cell stimulation. Int Arch Allergy Immunol 2002;128:15-23.
112. Li XM, Serebrisky D, Lee SY, et al. A murine model of peanut anaphylaxis: T and B cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol 2000;106:150-158.
113. Vader LW, Stepniak DT, Bunnik EM, et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology 2003;125:1105-1113.
114. Nelson HS, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 1997;99: 744-751.
115. Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003;348:986-993.
116. Del Guidice M, DeLuca MG. The role of probiotics in the clinical management of food allergy and atopic dermatitis. J Clin Gastroent 2004;38 (6 Suppl):S84-85.
117. Dugas B, Mercenier A, Lenoir-Wijknroop I, et al. Immunity and probiotics. Immunol Today 1999;20:387-390.
118. Paganelli R, Ciuffreda S, Verna N, et al. Probiotics and food-allergic diseases. Allergy 2000;57 Suppl 72:97-99.
119. Hiller R, Laffer S, Harnawegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB Journal 2002;16:414-416.
120. Malandain H. Vers une nouvelle generation de tests in vitro. Alim Inter 2002;7:151-153.
121. Vila L, Beyer K, Jarvinen KM, et al. Role of conformational and linear epitopes in the achievement of tolerance to cow’s milk allergy. Clin Exp Allergy 2001;31:1599-1606.
122. Jarvinen KM, Beyer K, Vila L, et al. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J Allergy Clin Immunol 2002;110:293-297.
