Pediatric Fever: Myths and Management
Author: Charles E. Stewart, MD, FACEP, Emergency Physician, Colorado Springs, CO.
Peer Reviewers: Ronald M. Perkin, MD, MA, Professor and Chairman, Department of Pediatrics, Brody School of Medicine at East Carolina University, Greenville, NC; and Christian Montagano, DO, Emergency Physician, Assistant Director of the Emergency Department, Our Lady of Lourdes Hospital, Camden, NJ.
One of the most common manifestations of disease, fever in children remains controversial, misunderstood, and often is thought to be mysterious. Fever has been recognized since antiquity and remains one of the most frequent complaints in emergency departments (EDs), physician offices, and clinics. Treatment of fever also is a lucrative business; an estimated $6 billion dollars is spent worldwide each year on antipyretic drugs.1,2
The clinician always should realize that fever is not a disease itself, but a manifestation of a number of different disease processes. Because there are substantial differences in the cause and outcome of fever-generating illnesses in children of different ages, the discussion must consider age. Not surprisingly, there is a diversity of approaches to fever.3-6 —The Editor
What Is Fever?
Fever is a state of elevated core temperature. It is a neuro-chemical response common to many animals and often is part of the defensive response to the invasion of microorganisms or inanimate matter recognized as alien or pathogenic by the host,7 making a strong argument that fever has a net benefit to the species.
Fever is found in almost all infectious diseases, but also occurs in neoplastic disorders, autoimmune disease, acute metabolic and endocrine disorders, granulomatous disorders, drug reactions, vascular thrombosis or infarction, and trauma. Unfortunately, fever is not the clearly defined concept that many believe it to be.
Definition of Fever
A temperature of 98.6°F is considered normal by some, but this doesn’t account for individual variations, environmental exposure, or the slightly faster metabolism of a child. Most definitions of fever are based on an 1868 study by Dr. Carl Reinhold August Wunderlich, which defined normal as 98.6 °F. However, this study and other such studies did not involve children and did not account for individual variations, environmental exposure, or the slightly faster metabolism of a child. Body temperature also varies during the course of the day, with the highest temperature during the late afternoon.8 Conversely, children often have their lowest temperature early in the morning. With teenagers, the menstrual cycle also causes a cyclic variation in the temperature. Finally, body temperature varies with the part of the body that is measured. “Core” organs such as the liver have a higher temperature than the extremities. In a cold environment or in response to a decrease in core temperature, the cutaneous blood flow normally decreases as a means of retaining heat within the body core.
Measurement of Fever
The rectal thermometer is perceived to be the gold standard for measurement of a temperature in children. The rectal temperature is obtained by placing a lubricated thermometer in the rectum. The usually accepted reference range is 36.1-38.0°C (97-100.3°F.) Rectal temperatures do not respond quickly to induced heating or cooling of the body. (See Table 1.)
Table 1.
Recommended Temperature
Measurement Techniques
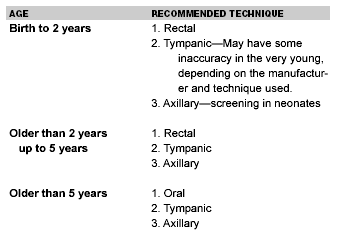
Oral temperatures usually are preferred in adults and children older than 5 years. Typically, a thermometer is placed under the tongue for four minutes when using a mercury thermometer or suitable substitute. The sublingual site is easily accessible and reflects the temperature of the lingual arteries. The oral temperature easily is influenced by the recent ingestion of hot or cold food and drink and by mouth breathing. Proper technique of oral temperature measurement includes having the child keep a sealed mouth, with the tongue depressed for about 3-4 minutes. This is not always attainable in a small child, an unconscious patient, or an uncooperative patient.
The accuracy of oral temperatures is somewhere between axillary and rectal temperatures. Generally, the older the child, the more accurate the measurement is. This may be due to better compliance on the part of the patient or better technique.
Pacifier thermometers are available, but have not been well evaluated for accuracy.9 The available data on these devices is quite limited. Electronic oral thermometers can ascertain the temperature more quickly. The usual oral normal reference range is 35.6-37.4°C (96.0-99.3°F)
Tympanic thermometers measure the thermal radiation emitted from the tympanic membrane (TM) and the ear canal. There are two techniques: measurement of the temperature by putting a thermocouple directly on the tympanic membrane or measurement of the reflected infrared radiation from the tympanic membrane. The former is potentially painful and invasive. It is not often used.
Tympanic thermometers that measure the infrared radiation from the eardrum also are called infrared radiation emission detectors (IRED). Because the amount of thermal radiation emitted is in proportion to the membrane’s temperature, IRED accurately estimates TM temperature.10 In contrast with other sites of temperature measurement, the TM’s blood supply is very similar in temperature and location to the blood bathing the hypothalamus, the site of the body’s thermoregulatory center. It is, therefore, an ideal location for core temperature estimation. Crying, otitis media, or earwax have not been shown to change tympanic readings significantly.11,12
Most models of tympanic probes use an offset or an internal calculation that transforms the measured ear temperature into a rectal equivalent or an oral equivalent. These offsets may represent a source of error. The formula often is based on adult data and subsequently is applied to all age groups. This formula may not apply to the child younger than 3 years. In one published study, this offset was adjusted, and the TM temperatures more closely matched the rectal temperature.13 The actual ear reading was found to indicate temperature reliably in premature and full-term neonates.14
The size of the temperature probe in children younger than 3 years may not be small enough to be placed correctly in the ear canal. For the sensor to detect heat from the drum, it must be placed so that the sensor records infrared reflection from the drum, not from the canal walls. This requires that the ear canal is straightened as when using an otoscope—the pinna pulled down and back from children younger than 3 years.
At present, there appears to be no consensus about how accurate the tympanic IRED can be in assessing temperatures in children younger than 3 years of age. Even one manufacturer-sponsored study felt that the devices often were inaccurate in children younger than 3 years.15 Tympanic methods correlate fairly well after the age of three months, but if there is any doubt, obtain a rectal temperature.
Axillary temperatures have a sensitivity of only 50-70% for detecting elevated temperature documented by rectal thermometers. Despite its demonstrated low sensitivity and specificity in detecting fever, axillary temperature is recommended by the American Academy of Pediatrics as a screening tool for the evaluation of fever in neonates because of a perceived risk of rectal perforation with a rectal thermometer.16 Axillary temperatures are useful only as a screening tool for hypothermia when dealing with multiple casualties.17 The reference range for axillary temperatures is 37-37.4°C.
Temporal artery temperatures can be taken by a noninvasive IRED that measures the temperature of the area around the temporal artery.18 As the blood flow to the temporal artery is similar to the blood flow around the hypothalamus, this technique has attractions. The temporal artery has limited sensitivity for detecting fever in infants, but it may be more accurate than a tympanic thermometer.19 It is better tolerated than rectal temperatures. Further evaluation of this technique is needed.
Forehead temperature measurement devices are less accurate than axillary thermometers and should be discouraged.
Parents may report a fever in their child by subjective information (touching the forehead or the torso). This parental reporting of fever has been shown to be a moderately reliable indicator of an actual fever.20 These patients may be afebrile when they present to the ED.
Although accurate temperatures may be important in helping make clinical decisions about required diagnostic procedures and subsequent treatments, the patient’s temperature is only one risk factor in assessing an ill child. EPs must exercise clinical judgment in providing optimal care to the child with fever, irrespective of the instrument used to obtain this measurement.
Mechanism of Fever
Fever is a regulated rise in the body temperature after an alteration in the body’s metabolic set point (at the hypothalamus) that is mediated by numerous endogenous and exogenous chemicals, including leukocytosis and phagocytosis. These chemicals induce the cyclooxygenase COX-2 activation of the arachidonic acid inflammatory cascade and enhanced biosynthesis of pro-staglandin E2 by the hypothalamic vascular endothelial cells.21
Fever is tightly regulated by the immune response.22 Although infections are the most common cause of fever in children, there are multiple other triggers for the acute phase response and subsequent fever. These include transfusion reactions, juvenile rheumatoid arthritis, malignancy (especially lymphoma and leukemia), pulmonary embolism, connective tissue diseases, inflammatory reactions of trauma, burns, medications (including antihistamines, some antibiotics, and an overdose of NSAIDs—particularly aspirin), immunizations, and dehydration.
Non-infectious causes of fever include:
- Environmental factors such as high external temperatures;
- Over-bundling of children during the winter months;
- Malignancy;
- Rheumatoid diseases;
- Certain drugs;
- Salicylates, in particular, can cause fever when they are ingested in overdose. Other less common ingestions that can cause fever include phenothiazines, antidepressants, atropine, amphetamines, cocaine, and anticholinergic medications;
- Recent immunizations;
- Diphtheria/tetanus/pertussis (DPT) immunization may cause fever within a few hours after the injection that may last as long as 48 hours;
- Measles/mumps/rubella (MMR) immunization can have delayed temperature elevations (up to 7-10 days after the injection);
- Teething. Fever associated with teething is usually low-grade.23,24 A fever higher than 102 should be investigated and may well be due to some other illness.25 Diarrhea, respiratory symptoms, pulling at the ears, high fever, or convulsions should not be attributed to teething and require medical attention;26
- Trauma and burns; and
- Tissue infarction.
Patterns of Fever
Febrile patients with localizing signs present few difficulties in diagnosis. Fever patterns are most helpful in diagnosing febrile illnesses that do not have localizing signs and may provide a clue for these difficult-to-diagnose patients. Use of antipyretics, steroids, and anti-inflammatory drugs with antipyretic properties all influence these patterns of fever.
Continuous Sustained Fever with Minimal Remissions (less than 2.0°F). This fever pattern is classic for the patient with lobar or gram-negative pneumonia, rickettsial diseases, typhoid fever, tularemia, and falciparum malaria.8
Intermittent Fever with Wide Fluctuations (Picket-fence fever, septic, quotidian). Intermittent fevers often are normal or low in the morning and peak between 4 and 8 PM. This group of fevers often includes localized pyogenic infections and bacterial endocarditis. Malaria often has a daily spike (quotidian), a spike every third day (tertian), or a spike every fourth day (quartan), depending on the species of malaria infecting the patient.8
Intermittent Hectic (Charcot’s) Fever. This pattern consists of sporadic episodes of fever followed by periods of normal temperature and then recurrence of fever. This is a frequent and reliable pattern in cholangitis.8 It often is associated with cholelithiasis, jaundice, and toxic appearance, but may occur in patients without jaundice.
Pel-Ebstein Fever. The Pel-Ebstein pattern of fever is fever of a week or longer followed by an equally long afebrile period and then repetition of the cycle.8 It can occur in Hodgkin’s disease, brucellosis, and relapsing fever. Occasionally, it can be found in tuberculosis.
Jarisch-Herxheimer Reaction. This is a fever associated with the treatment of primary or secondary syphilis, leptospirosis, and tick-borne relapsing fever. It occurs several hours after the beginning of antibiotics (penicillin) for these diseases. It is sometimes seen following tetracycline or chloramphenicol therapy for brucellosis.
Typhus Inversus Fever Pattern. The typhus inversus fever pattern is a reversal of the diurnal pattern of fever with the highest temperature occurring in the early morning hours rather than later in the afternoon or early evening. This pattern is found in miliary tuberculosis, salmonella, hepatic abscesses, and occasionally in bacterial endocarditis.
Saddle-back (Biphasic) Fever. This fever pattern is several days of fever, a distinct reduction in the fever for about a day, and then several additional days of high fever. This pattern is typical of dengue, yellow fever, Colorado tick fever, relapsing fever, Rift Valley fever, influenza, and other viral infections such as polio and lymphocytic choriomeningitis.8,27
What Is Not Fever
Fever is not the same as hyperthermia. Hyperthermia occurs when heat production exceeds the heat losses or when heat loss mechanisms are defective.28 Mechanisms for hyperthermia include neuroleptic malignant syndrome, malignant hyperthermia, and heatstroke.29 These diseases are medical emergencies that require rapid external cooling. They do not reset the thermostat, and the patient’s temperature will continue to rise until effective treatment is given. Only set point alteration fevers respond to antipyretics.
A common example of hyperthermia is heatstroke associated with exercise or exposure to extreme heat, but hyperthermia can occur with perspiration-inhibiting drugs and modest temperature elevations. The elevated temperature associated with heat illnesses (heat production/gain greater than heat losses) will not respond to ibuprofen, aspirin,or acetaminophen.29,30
As the core temperature rises, if the oxygen supply does not keep pace with the intracellular needs, the cells will become hypoxic and begin to die. In a conditioned athlete, the external heat load is dissipated well, and the heart and cardiovascular system can provide for the cardiovascular load of the heat dissipation, supply necessary oxygen and metabolic substrates, and meet the circulation needs of the exercise. In poorly conditioned, chronically ill, dehydrated, or very young patients, the cardiovascular system is not able to provide for the needs of both circulation and cooling.
As the temperature rises, the cardiovascular system becomes unable to both provide adequate circulation and continue cooling efforts at some point. The result is multi-system breakdown with diffuse cellular death. The cellular breakdown appears to be responsible for some of the delayed morbidity in those patients who survive the emergent phase of heat stroke. Survival from heat stroke depends on adequate response of the cardiovascular system, as more than normal cardiac output is needed to meet the elevated circulatory demands.31
Malignant hyperthermia is also not a fever. Malignant hyperthermia typically is due to a mutation in the calcium channel of the muscle sarcoplasmic reticulum. It most commonly occurs during the induction of general anesthesia with concurrent use of a depolarizing paralytic agent. The hyperthermia that ensues must be treated rapidly both with cooling and a calcium channel blocker with dantrolene to prevent further tissue damage.
Neuroleptic malignant syndrome is thought to be due to a tendency of some antipsychotic drugs to induce muscular rigidity and direct effects on hypothalamic heat-conserving mechanisms. Both of these effects are the result of blockade of dopamine receptors. Neuroleptically induced hyperthermia will respond to dantrolene and external cooling. (The antipsychotic agents also are implicated as a contributing factor for heatstroke.)29
How Hot Is Too Hot?
Modern clinicians generally subscribe to the notion that the febrile range has an upper limit, but there is great controversy about the precise temperature defining this limit. Animal studies suggest that a body temperature of greater than 107.6°F in humans may trigger enough adverse effects on a cellular level to cause death. These studies were done by injecting pyrogens or by artificially heating the animals. This is not the same as a naturally occurring fever, but is the closest available laboratory model.
Not all of this animal-derived data are supported by human clinical research. Healthy volunteers can withstand core temperatures of 42°C (107.6°F) for as long as 4 hours without any ill effects.32 Indeed, marathon runners and other skilled athletes engaged in strenuous exercise often will have higher temperatures with no evidence of any damage.31,33 The ability to tolerate a high temperature is multifactorial and depends in part on conditioning, general health, oxygen supplies, hydration, and innate physiological differences.
Why Be Concerned?
As noted earlier, fever is a common pediatric presentation to the ED. Nearly 30% of pediatric office and ED visits for children after three months of age are for fever.34 In most clinical situations, fever causes only minor discomfort, does no major harm, and actually may benefit the host defense mechanisms. Although fever usually is associated with a non-serious, self-limited disease, it also is a cardinal sign of a serious bacterial infection. Therein lies the crux of the problem.
Both health professionals and parents may believe in a lower standard for fever and react completely out of proportion to the severity of the illness. In a Johns Hopkins interview paper, 90% of parents felt that fevers were always a source of concern, 21% felt that a fever alone would kill a child, and 85% felt that a child should be wakened to administer an antipyretic.35 Fifty-two percent of these caregivers would check their child’s temperature at least every hour if the child had a fever. Interestingly, when compared with similar surveys in the 1980s, the parents today are in command of less correct information than those raising children in the ’80s.35
Fever must be considered in the context of the patient’s overall condition, age, and prior history. A 3-year-old child with a temperature of 104.5°F, industriously at play in the ED waiting room, has an exceptionally low likelihood of having serious illness. The limp and lethargic 4-month-old child with a temperature of 99.9 is critically ill.
Unfortunately, parents often are quite concerned about the fever and want ED clinicians to do something, a feeling, in part, driven by advertising. The manufacturers of acetaminophen and ibuprofen alike run ads encouraging parents to treat fever rapidly with implications that not to do so is inappropriate parental behavior.
Studies have shown that many caregivers believe fever may cause seizures, brain damage, death, dehydraton, coma, delirium, or blindness.35,36 Many caregivers feel that fever will continue to rise to potentially lethal levels if left untreated. These caregivers are unaware that the febrile response is homeostatic and the body does not allow fever to rise out of control to potentially lethal levels. Surprisingly, these beliefs are shared by some pediatric health care providers. A survey of 172 pediatricians found that 65% felt that fever alone could produce “serious complications” if not treated … and were unable to cite these serious complications.37 Pediatric emergency nurses were no more reliable, with almost one-third of polled pediatric ED nurses feeling that a fever of under 104°F was dangerous.38 Parents also may believe that every fever requires antibiotic therapy for their child. The emergency physician needs to invest time educating parents about the dangers of indiscriminant use of antibiotics.
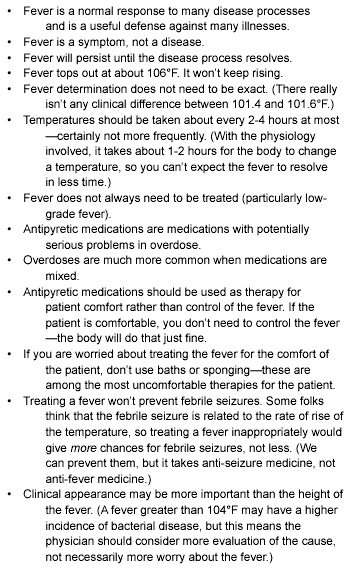
Obsession with a number can detract from the overall care of the patient. Fortunately, more discriminating and evidence-based practice patterns will decrease this pervasive fever phobia. The aware physician realizes how important it is to educate the parent and the health care provider alike that fever is a sign and a symptom, not a disease. Caregivers need to be educated that fever is a physiologic response to an insult that stimulates the body’s inflammatory defenses. (See Table 2.)
Table 2.
Thoughts to Share with Patients about Fever
There is no question that during hyperthermic disorders such as heat stroke and malignant hyperthermia, the core temperature can rise to levels that are lethal. Fortunately, fever and hyperthermia are two different disease processes.
The Risk/Benefit Analysis: Should Fever Be Treated?
Should fever be treated? The answer to this question is not easy and depends upon the goals of treatment. Although the complex biochemistry of antipyretics is becoming better understood, the indications for use clearly are not.
Two critical assumptions are made when prescribing an antipyretic. One is that the fever is, at least in part, noxious. The other is that suppression of the fever will reduce the fever’s noxious effects without harm to the patient. Neither of these assumptions has been validated adequately. In fact, consideration of the mechanisms of fever indicates that in the absence of brain injury, fever is a normal and adaptive physiologic response.39,40 As noted earlier, there is considerable evidence that fever is an important part of the body’s resistance to infection.
Beneficial Effects of Fever
Multiple studies have reported a benefit of fever on the overall outcome of infections.41,42 Fever can retard the growth and reproduction of bacterial and viral microorganisms, enhance neutrophil production and T-cell proliferation, and aid the body’s acute phase reaction. Fever was used to treat syphilis, and artificially induced hyperthermia has been used to treat gonococcal endocarditis. (Indeed, the Nobel prize was awarded for the treatment of neurosyphilis by induction of fever.)43
Numerous animal studies of infection show that antipyretic therapy increases the morbidity and mortality of the host.44 A high temperature has been associated with lower mortality in spontaneous bacterial peritonitis. Patients who have gram-negative bacteremia and an elevated temperature on the day of bacteremia have better survival.
The elevation of body temperature by a few degrees may improve the efficiency of macrophages in killing invading bacteria, while it impairs the reproduction of many micro-organisms, giving the immune system an adaptive advantage.39,40
Conversely, increased mortality is found in patients with sepsis, bacteremia, or meningitis who are unable to mount a febrile response. Gathering evidence points to IL1 and TNF-alpha (endogenous mediators of the febrile response) as contributing factors in the pathophysiology of bacterial sepsis, and possibly to the disability associated with other infections diseases.
Unfortunately, critical controlled studies are lacking that demonstrate that a low-grade fever can improve the outcome of infectious illness in humans.
Detrimental Effects of Fever
There are a few truly detrimental effects of the febrile response that often are not appreciated. The most persuasive evidence of the adverse effects of the febrile response appear in studies about gram-negative bacterial sepsis.42 In these studies, tumor necrosis factor and interleukin-1 induce fever, hypoglycemia, shock, and death. As noted above, both of these substances are cytokines that are elaborated by the febrile response. When animals develop induced tolerance to tumor necrosis factor, they are protected against the hypotension, hypothermia, and lethality of gram-negative bacterial sepsis.45 Similar data suggest that pyrogenic cytokines also may mediate some of the systemic and local manifestations of sepsis caused by gram-positive bacteria and some other infections in laboratory animals.45
Whether the febrile response may be beneficial or detrimental to the individual may be determined by the peak systemic concentrations of cytokines achieved during the infection. If the concentrations of the cytokines are low, the effects seem to be beneficial. If the concentrations are above a yet-to-be-determined critical level, the same cytokines may contribute to the damage caused by sepsis. It is, of course, difficult to separate the effects of the febrile part of the febrile response from the inflammatory cascade that is both cause and effect within the febrile response.
Fortunately for the clinician, these effects are relatively rare. The clinical implications of this data in human practice have not yet been determined. Indeed, there is no proof that these theoretical effects of fever actually contribute to an adverse clinical outcome of infections. At least one author proposes the hypothesis that the physiological response to sepsis accelerates the inevitable demise of a hopelessly infected and potentially contagious individual to limit the spread of infection within the species.45
The septic patient, therefore, may benefit in some cases from measures directed specifically against pyrogenic cytokines. Further research in this complex manifestation of the febrile response surely will be forthcoming.
Please note, however, that these detrimental effects of fever are not the effects seen in the vast majority of patients in the ED practice. They also are not the effects of fever feared by parents and practitioners alike and popularized by the manufacturers.
Detrimental Effects of Treating Fever
Giving an antipyretic can disrupt fever patterns. This important diagnostic sign is characteristic of only a few diseases and often based on geographically dependent epidemiology as previously discussed. Because only a few diseases have characteristic fever curves, the utility of this sign often is not appreciated by those who have not been involved in the diagnosis and treatment of these diseases.
Finally, fever is an important indicator of disease progression. Suppression of this fever may delay needed diagnostic studies or changes in antimicrobial therapy.
The Rationale for Treatment of Fever
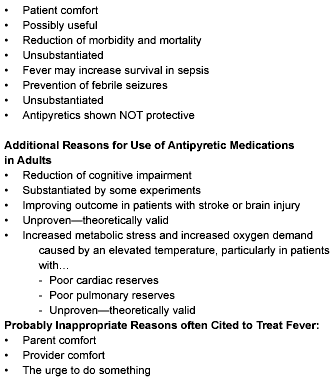
Theoretically valid reasons for treatment of fever include (see Table 3):
Table 3.
Reasons Cited for Use of Antipyretic
Medications in Children
• Increased metabolic stress and increased oxygen demand caused by an elevated temperature, particularly in patients with poor cardiac reserves or poor pulmonary reserves.
In fact, the metabolic and cardiovascular costs of fever are substantial, especially during the “chill” phase of the response with its shivering-induced increase in metabolic rate, peripheral vasoconstriction, and increased arterial blood pressure.22 Van’t Hoff’s rule states, in part, that the cellular metabolism increases about 13% for each centigrade degree rise in temperature. At 40.5°C (105° F), the cellular metabolism is 50% above normal. Although antipyretic therapy has theoretical merit in this regard, this theory has not been confirmed experimentally, even in patients with underlying cardiac and pulmonary diseases.21
• Patient comfort.
Antipyretic therapy often is advocated to make the child comfortable. Carefully controlled studies of efficacy have not been able to give any indication of how much comfort is provided by fever control.46 The general experience seems to support this rationale, however.
Febrile patients feel ill not simply because they have an elevated temperature. In fact, an elevated body temperature may not be particularly troublesome—as evidenced by the wide popularity of hot tubs and saunas. Anecdotally, multiple running, playing children with temperatures as high as 104°F in many EDs attest to the fact that fever alone may not be a comfort crisis.
Much of the discomfort surrounding illness comes from symptoms other than the fever. Generalized symptoms such as anorexia, headache, nausea, malaise, myalgias, and back pain often accompany the fever as constitutional symptoms. Since all of the antipyretic agents also are analgesic agents, many of these symptoms will be relieved by the use of antipyretic agents.
• Reduction of morbidity and mortality.
• Fever may increase survival in sepsis.
This point has been addressed previously in the discussion about mechanism of fever. Only one randomized study in humans has looked at survival of septic patients treated with antipyretics. This study found that antipyretic therapy with ibuprofen did not improve survival in patients with sepsis. Recent data demonstrating fever-induced expression of several heat-shock proteins that are protective against sepsis raise the concern that antipyretic therapy actually may potentiate the adverse effects of sepsis in some patients.47
• Fever may shorten viral illnesses.
This point also has been addressed previously in the discussion about mechanism of fever. The use of antipyretic treatment may have an adverse effect on the human immune system. Adults infected with rhinovirus treated with either aspirin or acetaminophen had increased and more prolonged viral shedding than the placebo control group. Children with varicella who are treated with acetaminophen have been shown to have a longer duration of lesions than a placebo group.48 Recent reports also have shown enhancement of resistance to viral and bacterial infections by pyrogenic cytokines.49,50
Additional reasons for use of antipyretic medications in adults • Reduction of cognitive impairment.
Antipyretic therapy might be beneficial in reducing the confusion, dementia, and mental dysfunction that sometimes is associated with fever. In one study, fever-associated cognitive impairment was reduced with aspirin.51 In another study, increased anxiety and depression together with worsened memory were noted in patients with laboratory-induced fever relative to control patients.52 Unfortunately, this study did not look at the use of antipyretic therapy to decrease such symptoms.
Possibly inappropriate reasons often cited to treat fever:
- Parent comfort;
- Provider comfort; and
- The urge to do something.
Despite the pervasive application of antipyretics by physicians, nurses, pharmacists, and parents, it remains unclear whether reducing the core temperature actually will benefit the febrile patient.53 This feeling of the importance of treatment of the fever is pervasive.
Indeed, in several studies, the speed at which the fever fell was considered an important variable.54-56 In one study, the caregivers would awaken their child to take a temperature every hour or less and give antipyretics during a febrile illness. This is certainly not looking out for the patient’s comfort.35
The patient’s real disease will not disappear just because the fever has been treated, no matter how reassuring this is to provider, nursing staff, and parent alike. Indeed, a small older pediatric study showed that appropriate changes in antibiotic regimens were delayed for children who received antipyretics compared to those who had none.57
The wise emergency provider always realizes that they are never treating just a child, but also treating the parents. Indeed, that provider also may be treating him/herself and/or the nursing staff when faced with a febrile child. The physician may realize that fever is not the disease, but remain under significant pressure from multiple sources to make the child better by treating the fever.58 (A similar battle is being waged about the inappropriate use of antibiotics for minor upper respiratory illnesses.)
• The possibility of febrile seizures.
Children between 3 months and 5 years have an incidence of seizures during episodes of fever at a frequency of between 2 and 5% in the United States and Western Europe.59 The majority of children with febrile seizures have temperatures above 39.0°C at the time of their seizure. A common myth is that treating a fever can prevent a febrile seizure. This myth is often cited by parents of children who have had a febrile seizure and may be echoed by nursing staff.
Unfortunately, there is no evidence to show that antipyretic therapy is effective in prevention of these seizures.60 More recent studies have shown that it doesn’t matter whether acetaminophen is given in moderate doses or in high doses. It still fails to reduce the rate of recurrence of febrile seizures.61-63 Ibuprofen also fails to prevent recurrent febrile seizures.64 There is simply no evidence that bringing the fever down by any means will stop or prevent a febrile seizure. The practice of giving acetaminophen or ibuprofen around the clock is not supported in the literature and may contribute to parental fever phobia.
• The child will have (brain or other organ) damage from the fever.
(This point has been previously discussed and there is little clinical validity to this argument. The emergency physician should carefully separate the consequences of fever from those of hyperthermia.)
• The height of the fever is a marker for serious illness. (This may or may not be true in the post-haemophilus influenza immunization era.)
Numerous early investigators of the consequences of fever have noted that there is a significant correlation between the height of fever and the incidence of serious bacterial infections in children.65-69 In these studies, the likelihood of such bacterial infections increases sharply in children with a temperature greater than 40°C. These studies may or may not continue to be valid in the face of newer epidemiology caused by the immunizations currently recommended and offered to children. The emergency physician should have a heightened suspicion for bacterial illness in these patients, but also should remember that these studies were conducted prior to institution of haemophilus influenza immunization. The emergency physician also should be aware that many viral illnesses can provoke high fevers. While the height of the fever has some positive correlation, the cogent emergency physician must realize that it is an inefficient discriminator as some children with serious bacterial illness will not have an elevated temperature.
Many investigators have suggested that the response of a fever to the administration of an antipyretic may be an important diagnostic maneuver. “When the fever falls and the child looks better, then that illness is not serious.” There are two parts to this myth:
• Resolution of the fever with antipyretics will not indicate that the illness is less serious.
Unfortunately, resolution of fever does not appear to be an appropriate diagnostic maneuver when the response of children with bacteremic and non-bacteremic infections are compared. Of six such investigations in recent history, only one found that there was a difference in the response of children with bacteremic or non-bacteremic fever.70-75 Unlike the five prospective investigations that showed no difference, this single study was retrospective. In short, fevers due to serious infections are just as responsive to antipyretic therapy as innocuous infections.
Resolution of the fever with antipyretics is thought by some to be an important discriminator in the treatment decision of the child. Unfortunately, the efficacy of antipyretics as a risk stratification factor for the presence of serious bacterial illness in the febrile child has never been proven. In fact, in at least one very well done study, the reaction of the child to an antipyretic was unable to distinguish between children with non-bacterial infections, meningitis, and bacteremia.76 Failure of antipyretics to control the fever has not been proven to correspond with the severity of the illness.74
• Improved appearance of the child (independent of the child’s temperature) after administration of antipyretics does not mean that the illness is less serious.
As noted above, all of the antipyretic medications have additional actions, including pain relief, that can make the child look better after their effects. There aren’t any data showing that improvement of the child is an accurate discriminator of the seriousness of the illness.71,73,77,78(Indeed, the data cited above about the treatment of the temperature argue eloquently that the serious disease process can respond, for a time, to the effects of antipyretic medications.)71
Indeed, evaluation of the child prior to the reduction of the fever correlated better with the presence of a serious bacterial illness than after treatment with an antipyretic.73 Treatment of the fever, with or without improvement of the child, has not done anything to treat or diagnose the process that is causing the fever. Fever is a symptom, not a disease.
Approaches and Mechanisms to Treat the Fever
As this article has previously pointed out, treatment of the fever should be considered in a completely different light than treatment of the disease that is causing the fever.
Various treatments have been used to treat fever for well over two millennia. The marketplace is now replete with drugs capable of suppressing fever. The following treatments have been currently recommended and are discussed in detail:
- Antipyretics to reset the set point;
- Acetaminophen;
- Ibuprofen;
- Aspirin;
- A combination of antipyretics;
- Alternative medications;
- Environmental manipulation;
- Sponging; and
- Undressing the patient.
Antipyretics. The drugs most commonly used today to suppress fever are acetaminophen, ibuprofen, and other non-steroidal anti-inflammatory drugs (NSAIDs), and the salicylates (sodium salicylate and acetylsalicylic acid -ASA).30 Acetaminophen, aspirin, and the NSAIDs all seem to block the conversion of arachidonic acid to prostaglandin E2 by inhibition of cyclooxygenase (COX) 2. Production of PGE2 at sites within the hypothalamus is widely thought to be a critical step in the process responsible for raising core temperature after the febrile response is activated.21
COX-1 has three folding subunits: a growth-factor-like domain, a membrane binding section, and an enzymatic domain. COX-1 sites are probably the cause of gastrointestinal side effects such as damage to the stomach lining.
The structure of COX-2 closely resembles that of COX-1. The active site of COX-2 is a little larger and can accommodate larger structures than that of COX-1. COX-2 is induced by inflammatory stimuli and by cytokines. COX-2 sites are probably the source of the anti-inflammatory actions of acetaminophen and the NSAIDs.
Antipyretics block or reverse fever’s cytokine-mediated rise in core temperature but do not affect the body temperature in the afebrile state. The reader needs to be aware that antipyretic therapy is not indicated for the treatment of exogenous hyperthermia (such as heat stroke), and is potentially dangerous.
The duration of action of an antipyretic drug depends upon both its concentration at the site of action and whether it is a reversible or an irreversible COX inhibitor. Because aspirin is an irreversible COX inhibitor, its antipyretic activity will persist until new enzyme is generated at the site of action. Other NSAIDs are reversible inhibitors of COX and have activities that vary with the concentration at the site of action.
There is a delay between the time that an antipyretic agent is administered, absorbed, reaches its site of action, and the time that the core temperature begins to fall. This antipyretic latency period also might be influenced by the capacity of arachidonic acid metabolites (such as PGE2) to decrease the production of pyrogenic cytokines. This delay explains why the maximal effect of the antipyretic agents occurs some three to four hours after oral administration.
Studies of the relative effects, potencies, and timing of administration of the various types of antipyretics have involved multiple clinical settings, numerous dosage patterns and formulations of the antipyretic agents, and differing measures of clinical efficacy. As a result, comparison of these agents in a comprehensive meta-analysis is not possible.
Even though these studies cannot be combined into a meta-analysis, there are several studies that compare ibuprofen with acetaminophen in children that are useful to examine.55,79-86 These studies suggest that ibuprofen is possibly more potent than acetaminophen when both are administered by the oral route. The difference in the effects of the two drugs is small, and they have a similar time course.
There is little evidence to support one antipyretic over another when considering effectiveness of the medication in treatment of fever.55,84,87,88 No evidence exists that delivery by either rectal or oral is more effective than the other. In the United States, there is no injectable antipyretic. (Ketorolac [Toradol] does have significant antipyretic properties, but is not approved for this indication—see comment about ketorolac below.)
Aspirin. Ancient Assyrian, Egyptian, and Greek physicians all exploited the antipyretic properties of extracts of the bark of the willow tree (Salix alba).88 Applying Peruvian cinchona bark as an antipyretic dates to the early 1600s.90 In 1763, Reverend Edward Stone, suffering from a shortage of cinchona, described the clinical benefits of willow bark to the Royal Society of London.91,92 Although his finding appeared novel, it simply confirmed the ancient physician’s observations.
Eighty years later, as related in an article on the history of antipyretic agents, salicylic acid first was prepared from a glycoside component of willow bark by Dr Piria.93 Synthesis of salicylic acid often is credited to Kolbe and Lautemann in 1874, but probably belongs to Gerland who synthesized it in 1852.94 Felix Hoffman developed acetylsalicylic acid during efforts to find a better tasting form of salicylate. Bayer introduced the new drug as Aspirin in 1899 and sales have been brisk ever since.
Aspirin was implicated by epidemiologic studies in the 1980s to as a cause of Reye’s syndrome in children.95-97It is no longer recommended as an antipyretic in children in the United States. (It still is used as a children’s antipyretic in several European and South American countries.)
Acetaminophen (Tylenol). Acetaminophen is a para-aminophenol derivative that inhibits cyclooxygenase and the formation and release of prostaglandin. It is absorbed in the gastrointestinal tract and reaches peak concentration within 30-60 minutes. Adverse reactions include allergic reactions and hepatotoxicity following overdose. Acetaminophen is an excellent antipyretic available in multiple concentrations, flavors, and dosage forms.
The use of acetaminophen for fever is relatively recent. Although precursors of acetaminophen such as acetanilid and phenacetin were developed in the early part of the 19th century, acetaminophen was not used as an antipyretic or analgesic until the 1950s.93,98 Phenacetin, a once-popular antipyretic and analgesic, fell out of favor because of a variety of reported side effects, including hepatic toxicity and nephrotoxicity.99 Because of the fewer side effects and less severe manifestations of these side effects, acetaminophen replaced phenacetin as an analgesic and antipyretic. Popular use of acetaminophen as an antipyretic and analgesic has blossomed since the 1950s.
Explanation of the antipyretic and analgesic activity of acetaminophen is thought to be based on tissue specific COX inhibition that is not found with the NSAIDs. Acetaminophen easily penetrates the blood-brain barrier and achieves cerebrospinal fluid levels that are comparable to those in the serum.100 Acetaminophen reduces the production of prostaglandins in brain preparations more potently than it does in other tissues.101 Indeed, central nervous system levels of PGE2 rise during fever and fall to normal levels when acetaminophen is given.102
Acetaminophen has a relatively weak activity against peripheral COX, and thus acts primarily in the central nervous system to reduce fever. This weak peripheral activity accounts for some of the poor anti-inflammatory action of acetaminophen. (Acetaminophen is only 5% as effective as aspirin in inhibition of peripheral COX.)103,104
Despite multiple literature recommendations, popular and professional acceptance, and wide media coverage of the use of acetaminophen to treat fever, at least one literature review and meta-analysis notes that there is a paucity of hard data that actually support the clinical utility of acetaminophen to treat fever.46 The authors noted that although acetaminophen is significantly better than placebo at resolving fever within 2 hours, the overall time to resolution of symptoms did not significantly differ between the two groups. Evaluation of defervescence at 2 hours comparing acetaminophen vs. cooling yielded inconsistent results. Meta-analysis showed no significant difference in adverse events between the comparison groups.
The average maximum decrease of the fever is about 1.6 to 2.0 °C when using the usual dose of 10-20 mg/kg every 4-6 hours. The peak effect occurs at 2 hours with 10 mg/kg dose and 2-4 hours with 20 mg/kg doses. There are multiple brand names, concentrations, and flavors of acetaminophen.
Ibuprofen (Advil, Motrin). Ibuprofen is a non-steroidal anti-inflammatory propionic acid derivative that also inhibits the biosynthesis of prostaglandin. It is absorbed in the gastrointestinal tract and reaches its peak plasma concentration in 2 hours. As with acetaminophen, metabolism takes place in the liver.
Adverse reactions include allergic reactions, exacerbation of asthma, renal toxicity, and gastrointestinal irritation.
Ibuprofen is an excellent antipyretic, available in multiple brands, concentrations, and flavors. This drug works slightly faster than acetaminophen and may be somewhat better at fever reduction. The difference in potency is very small, and the two agents have a similar time course with the maximum activity occurring 3-4 hours after administration.21
A 10 mg/kg dose usually is effective for 6-8 hours. There is scant data for use of this drug in children younger than 6 months of age. The parents/caregiver should be counseled against misuse, overuse, and frank overdoses of ibuprofen.
Other NSAIDs. Both pediatric and adult studies of the relative activity of other NSAIDs are sparse. Ketorolac is the only NSAID that can be given intramuscularly or intravenously. It is not indicated for fever, but has been studied, at least in adults, in the treatment of acute fever in the ED.106,107 This drug shows some promise in the treatment of fever in patients with intractable vomiting and diarrhea. An intravenous antipyretic possibly may be appropriate in these patients if treatment of the fever is deemed appropriate.
Overdose and Toxicity of Antipyretics. In addition to excessive fever monitoring, caregivers may be dangerously liberal with their antipyretic medications. Caregivers may give antipyretic medications for frankly normal temperatures and may give medicines at inappropriate doses or intervals. The parents/caregiver should be counseled against misuse, overuse, and frank overdoses of acetaminophen or ibuprofen. The risk for iatrogenic overdose with antipyretic agents is increased when:
- the caregivers are confused by the dosing information given. This is particularly true when multiple agents are recommended at multiple times as discussed above;108
- formulation-specific differences in acetaminophen preparation are not considered;
- adult-strength formulations are used for children;
- sustained-release preparations are administered at dosing intervals less than indicated on the product label;
- caregivers feel that the initial attempts at antipyretic therapy are ineffective and increase the dose under the principle that “more is better.” Remember that the maximum effect of antipyretics in common use is between 3-4 hours by the oral route;
- knowledgeable healthcare providers react inappropriately by treating playful, nontoxic children with low grade temperatures with antipyretic agents in EDs. This gives parents the impression that the fever is the disease.
Toxicity of Aspirin. Aspirin can cause fever in overdose. Aspirin is a relatively non-selective COX inhibitor and can have significant gastrointestinal irritation, even in quite modest doses.
As previously noted, aspirin was implicated by epidemiologic studies in the 1980s as a cause of Reye’s syndrome in children.95-97 For this reason alone, parents should be counseled to avoid aspirin as an antipyretic in children, since so many febrile episodes are due to viral illnesses.
Toxicity of Acetaminophen. Because acetaminophen has little peripheral COX inhibition, there are few reports of either gastric or renal toxicity.
While acetaminophen usually is metabolized by glucuronidation and sulfation, in excess, it is metabolized by the p450 2E1 pathway to N-acetyl-p-benzoquinoneimine (NAPQ1). Normally NAPQ1 is conjugated to glutathione. If NAPQ1 is produced in excess during the metabolism of acetaminophen, acute hepatotoxicity results. If glutathione stores are depleted, the risk of acetaminophen-induced hepatic toxicity is markedly increased.109 Concomitant use of medications, such as rifampin and phenytoin that induce the cytochrome P-450 enzyme system may potentiate toxicity through enhanced metabolism of acetaminophen to NAPQ1.
While the risk of hepatic toxicity in the setting of a massive overdose is well known, only recently has the literature addressed the risk of hepatic injury in doses that are at or slightly above the recommended ranges (4 grams in 24 hours). The results of one study suggest that acetaminophen may be toxic in doses as low as 1.7 times the manufacturer’s recommended dose and near those doses often prescribed for fever relief.110 In a recent series involving 71 cases of acetaminophen-induced liver damage, about one-third of the cases resulted from accidental overdoses in patients using the drug for pain relief.111
Multiple reports of children with significant hepatotoxic effects, including death, after unintentional overdoses at the hands of parents and other caretakers underscore the potential problems associated with this agent.112,113 In most of these accidental overdoses, infants and children are febrile and acutely malnourished. Reduction in caloric or protein intake, combined with multiple doses of acetaminophen can have significant effects in children.
The number of cases reported in the literature is small when compared to the total number of doses of acetaminophen administered. These reports probably are far less than the total number of cases of hepatotoxicity due to this drug. Presumably, other less severely affected patients have not been reported or even diagnosed. Parents should be advised about the potential hepatotoxicity of acetaminophen when given to children that exceed the weight-based recommendations. This is particularly important in the management of the child who is not eating because of the illness.
Toxicity of Ibuprofen. A number of adverse effects has been attributed to NSAIDs, including ibuprofen. The most important of these are renal dysfunction and gastrointestinal irritation/bleeding.87,114These adverse effects are a direct byproduct of their ability to inhibit COX.
Renal impairment in NSAID users occurs primarily among patients with pre-existing disease or other conditions associated with low intravascular volume or low cardiac output.114 Acute renal failure after ibuprofen overdose and interstitial nephritis after ibuprofen used in clinical doses has been described in patients without pre-existing renal disease.
A number of reports have linked ibuprofen use to renal complications in children, and three have described renal failure in children younger than 15 years who were treated with ibuprofen in therapeutic doses.114 Unfortunately, case reports don’t give any estimates of the rate at which renal failure occurs during treatment of children with short-term ibuprofen therapy for fever.
In a large survey examining antipyretic drug toxic effects, Lesko and Mitchell randomized more than 84,000 to one of two doses of oral ibuprofen or acetaminophen and later queried patients about adverse medical effects.115 The median duration of treatment for the fever was 3 days. Of 55,785 patients dosed with recommended doses of ibuprofen, four children developed gastrointestinal bleeding. There were no episodes of Reye’s syndrome, anaphylaxis, or acute renal failure among these patients. Autret and his colleagues also found an increase in adverse effects with ibuprofen compared with acetaminophen.82 No children had renal failure in this study. Children with serious dehydration, pre-existing renal, endocrine, or neoplastic disease were excluded from the study due to concerns that these children would have a higher incidence of renal failure.
The number of cases reported in the literature is small when compared to the total number of doses of ibuprofen administered. These reports probably are far less than the total number of cases of transient nephrotoxicity or gastrointestinal bleeding/erosions due to this drug. Presumably, other less severely affected patients have not been reported or even diagnosed. Parents should be advised about the potential nephrotoxicity and potential for gastrointestinal irritation of ibuprofen when given to children that exceed the weight-based recommendations.
Alternative Medications. Herbs, vitamins, supplements, homeopathic remedies, and acupuncture all have been used by caregivers and alternative health care providers to treat fever. Western herbalists use tea preparations containing herbs such as bupleurum root or boneset to reduce fever.116 Mild herbs such as peppermint, elderflower, or yarrow are recommended to provide comfort to the child who has a mild fever. Other remedies include elder tea, cinnamon, coriander, feverfew, and ginger. There is no set of randomized, controlled studies that address these alternative medications.116
Corticosteroids also are effective antipyretic agents, but their adverse side effects and effects on the host immune system make them undesirable for antipyretic agents.
Is a Combination of Acetaminophen and Ibuprofen Better Therapy? Parents often are instructed by healthcare providers that although either ibuprofen or acetaminophen is quite effective, the combination of the two will be more effective than either drug given alone. These healthcare providers teach that alternating ibuprofen and acetaminophen will provide better fever reduction than one drug alone. These practitioners also feel that the additive antipyretic effects will lead to a faster defervescence than either drug alone can provide. (Interestingly, 70% of clinicians in practice for fewer than 5 years endorse this practice, but only 45% of those in practice for more than 5 years will use this practice.)117 The use of such a combination often results when the desired therapeutic response to the initial dose of antipyretic is not observed shortly after administration of the drug.
The American Academy of Pediatrics often is cited as the source for this dosing technique, although the AAP has never endorsed this practice. A computerized search of the medical literature for scientific data supporting the safety and efficacy of this practice was conducted without any success. A single article in eMedicine advocated this approach, but offered no evidence to support the practice.118 (None of the references cited in this electronic article offered scientific data that supported the practice.) Other writers have contacted the Food and Drug Administration, and they disapprove of the use of this combination.119 McNeil pharmaceutical company was contacted by Mofenson and colleagues, and the company disclaimed and disagreed with this combination practice.120
Both acetaminophen and ibuprofen may have renal toxicity. Acetaminophen accumulates in the renal medulla, and its metabolites can cause cellular necrosis when glutathione is low or absent. Ibuprofen reduces renal blood flow by blocking production of renal prostaglandins and inhibits glutathione production.121,122 These effects may be synergistic.112,123
Future Antipyretic Therapy. Cyclooxygenase (COX)-2-selective inhibitors are attracting the attention of pharmaceutical companies for their ability to suppress fever. These companies are working on second- and third-generation derivatives with more potent activity against COX-2, some of which can be administered parenterally.1124 Preliminary data indicate that these agents may well have substantial antipyretic activity as well as anti-inflammatory activity.
Efforts to reduce the toxicity of antipyretic agents have taken a number of different clinical approaches. This includes the use of enteric coating, parenteral and rectal administration, and concomitant administration of H2-receptor antagonists.
Perhaps the most important task for the future is to develop more intelligent criteria for the use of antipyretic therapy. To do so requires research into the risk of fever, benefits of fever, and the actual benefits of the various modes of therapy that have been used to treat the fever.
Environmental Manipulation. Physical methods of lowering temperature are based on loss of body heat through evaporation, radiation, convection, or conduction. Other environmental methods include air conditioning, hypothermic (cooling) blankets, and fans. These physical methods may be life-saving for patients with heat stroke or with malignant hyperthermia. Antipyretic agents are the preferred method for fever reduction when such therapy is indicated. Physical methods of lowering temperature are preferred for the treatment of hyperthermia, heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome.
Two questions that remain to be answered about external cooling methods include: Is the discomfort of physical cooling in young children justified by any reduction in the complications of a high fever such as incidence of seizures? Is external cooling associated with lower morbidity than is treatment with antipyretic drugs alone? The latter question is provoked by the unwanted side effects of all of the physical methods of cooling including induction of shivering, hypermetabolic activity, and sympathetic activation.
External Cooling. External cooling has been used since antiquity to treat fever in both adults and children. Prior to his death in 323 BC, Alexander the Great suffered from a febrile illness that his physicians treated with cool baths.93 It continues to be employed in intensive care units to treat both children and adults with fever. A wide variety of techniques are used for external cooling. These include sponging with multiple different fluids, application of cold packs or ice, and exposure to wind blown from fans (often in conjunction with sponging).
In contrast to antipyretic drugs, external cooling lowers the temperature of febrile patients by overwhelming the body’s ability to produce heat. Unless antipyretic agents are given to lower the hypothalamic set point, or shivering is inhibited by other pharmacology, the patient will begin using every body defense (including shivering) to maintain body temperature.
When external cooling is used, evaporation and convection (a continuous spray of water combined with a relatively high velocity fan blowing warm air) will provide, perhaps, the most rapid cooling. This is the basis of the Body Cooling Unit proposed by Khogali.125
Sponging/Baths. Multiple physicians, nurses, and caregivers have recommended sponge baths for children. These have ranged from ice water baths, alcohol-water baths, and tepid water baths. Although the use of a water bath or sponging to cool the child appears to be a rapid way to reduce the child’s temperature, the result is quite counterintuitive.
Iced water baths are significantly less comfortable and are tolerated poorly by sick children, but are more effective at actually cooling the patient. While less effective in lowering febrile temperatures, sponging with tepid water has been reported to offer greater comfort than sponging with either ice water or alcohol in water.126
Before the 1950s, sponging was performed with alcohol (isopropyl or ethyl) mixed with the water or used alone to more rapidly cool the patient. Alcohol has the potential to cause dehydration and hypoglycemia in children, particularly in young children, and should not be used.127 Some children developed profound hypoglycemia, subsequent coma, and died.128-132 Despite these recommendations, alcohol still is used in certain communities in the United States and morbidity continues.119,133
Two large randomized trials compared the use of antipyretics and sponge baths for the treatment of young children with temperatures over 38.9°C.134-135 Both trials unequivocally demonstrated the superiority of antipyretic drugs for the reduction of temperature. Differences in patient comfort were not assessed in these studies.
How about using sponge baths and an antipyretic? Unfortunately, multiple studies also have shown that there is no significant difference in temperature at one hour between children treated with antipyretics and children treated with sponging and antipyretics.124,136The studies did show that there was a transient rapid lowering of the temperature in the first half-hour. Indeed, in one such study the children had a rebound increase in temperature after the bath was terminated.137 Newman found that tepid water baths combined with acetaminophen were no more effective than acetaminophen alone.
The use of sponging and/or water baths might be justified if, in fact, it decreased the rate of febrile seizures in children. Since acetaminophen does not protect against seizures and antipyretics cool more efficiently, it is unlikely that the addition of sponging would provide any such decrease in the incidence of seizures. There is, however, no study that proves just this point.
Water baths and sponging are uncomfortable for children. There appears to be increasing discomfort with cooler bath water. If this discomfort actually achieved an objective, then the discomfort could be tolerated as a necessary evil in the care of the patient. (Paradoxically, a major cited reason for treatment of fever is patient comfort.) Despite abundant evidence that shows there isn’t any significant difference between the use of water baths in the treatment of fever and the use of antipyretics alone in the treatment of fever, many physicians continue to recommend this treatment. There simply doesn’t seem to be any justification for this outdated practice in the treatment of fever in children. This is not true for the treatment of environmental hyperthermia.
Hypothermia Blankets. Hypothermia blankets are not often used in children. In a prospective study of adults treated in an ICU, hypothermia blankets induced wider fluctuations and more episodes of hypothermia than antipyretics alone.138 In comparison with usual antipyretic agents, they added no significant cooling effect to the antipyretic effects. They are not recommended for children for this reason. Use of hypothermia blankets was typically initiated by nursing staff, often without the knowledge of physicians. This made interpretation of antibiotic effectiveness and patient progress more difficult.
Evaporative Cooling. Evaporative methods of cooling have been thought to be some of the most effective means of promoting heat loss in the febrile patient. These methods often are thought to be the least likely method to induce shivering. There is no comparative trial that establishes this or any other methods of external cooling as superior.
Undressing the Patient. Perhaps the most physiologic treatment for fever is to simply undress the child. With the increased surface area to mass ratio of the child, radiation loss of heat will rapidly lower a temperature with few side effects or complications.
A common myth is that the patient should be carefully covered with blankets if chills are present. Unfortunately covering up only keeps in the heat. Chills are evidence of the hypothalamus causing the body to generate heat to reach the new set point.
Age-directed Approach to Evaluation of the Disease Causing the Fever
The physician needs to separate the assessment of the disease that is causing the fever from the fever itself. Because the cause of fever in children of different ages has different causes and consequences, the evaluation of possible diseases should be, in part, age-related. Multiple protocols for evaluation of the febrile child have been developed and are vigorously defended by each of their proponent groups and agencies. Each of these protocols is based on the statistical inference that since there is a percentage of children/infants (or even adults) that will have a serious bacterial illness that manifests itself with the first and only symptom of fever, all of the children/infants/adults should be subjected to a certain level of scrutiny (with radiographs/laboratory testing/cultures/ in-hospital observation) and therapy (observation/hospitalization/ antibiotics) to eliminate or reduce the missed percentage. Each of the proponent groups has its own level of comfort with their practitioners and the chance of missed serious illness and this comfort level drives the resulting protocol.
Summary
There is no question that fever remains a source of great concern to parent and physician alike. Parental fear of fever is certainly responsible for many ED visits and phone calls requesting advice and diagnosis. These concerns focus the parents on fever control and away from the more important issue—that fever is the symptom of a disease process. This inappropriate focus has been augmented by a multi-million dollar industry and its advertising intended to convince parents of the importance of reducing fever.
Fever during systemic infections or inflammation is a normal adaptive response to circulating cytokines. The intrinsic mechanisms that produce the coordinated autonomic, endocrine, and behavioral response of fever appear to depend on systemic inflammatory mediators as neuro-modulators in the central nervous system to regulate the acute phase reaction of fever. The antipyretic medications all block the COX pathway of inflammatory response.
The physician should document a clear need for the use of antipyretic medication (just as with any other medication). Use of multiple medications is not supported by the manufacturers, let alone the data. All fevers should be evaluated as extensively as necessary to identify a site or source of infection.
References
1. Garner A. Adaptation in the pharmaceutical industry, with particular reference to gastrointestinal drugs and diseases. Scand J Gastroenterol 1992;193 Suppl:83-89.
2. Anti-arthritic medication usage: 1991. Stat Bull Metrop Insurance Co 1992;73:24-25.
3. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
4. Isaacman DJ, Kaminer K, Veligeti H, et al. Comparative practice patterns of emergency medicine physicians and pediatric emergency medicine physicians managing fever in young children. Pediatrics 2001;108:354-358.
5. King BR, D. MM, Singer JI. The febrile child: The great debate. Paper presented at: 1998 ACEP Scientific Assembly, 1998; San Diego.
6. Kramer MS. The young febrile child: Evidence-based diagnostic and therapeutic strategies. Emergency Medicine Practice 2000;2:1-24.
7. Adam HM. Fever and host responses. Pediatr Rev 1996;17:330-331.
8. Mackowiak PA, Bartlett JG, Borden EC, et al. Concepts of fever: Recent advances and lingering dogma. Clin Infect Dis 1997;25:119-138.
9. Press S, Quinn BJ. The pacifier thermometer: Comparison of supralingual with rectal temperatures in infants and young children. Arch Pediatr Adolesc Med 1997;151:551-554.
10. Chamberlain JM, Terndrup TE, Alexander DT, et al. Determination of normal ear temperature with an infrared emisssion detection thermometer. Ann Emerg Med 1995;25:15-20.
11. Terndrup TE, Crofton DJ, Mortelliti AJ, et al. Estimation of contact tympanic membrane temperature with a noncontact infrared thermometer. Ann Emerg Med 1997;30:171-175.
12. Childs C, Harrison R, Hodkinson C. Tympanic membrane temperature as a measure of core temperature. Arch Dis Child 1999;80:262-266.
13. Brennan DF, Falk JL, Rothrock SG, et al. Reliability of infrared tympanic thermometry in the detection of rectal fever in children. Ann Emerg Med 1995;25:21-30.
14. Treolar D, Muma B. Comparison of axillary, tympanic membrane, and rectal temperatures in young children. Ann Emerg Med 1988;17:435.
15. Petersen-Smith A, Barber N, Coody DK, et al. Comparison of aural infrared with traditional rectal temperatures in children from birth to age three years. J Pediatr 1994;125:83-85.
16. Kresch MJ. Axillary temperature as a screening test for fever in children. J Pediatr 1994;104:596-599.
17. Stewart CE. Generalized hypothermia. In: Stewart CE, ed. Environmental Emergencies. Baltimore: Williams and Wilkins; 1990:91-118.
18. Roy S, Powell K, Gerson LW. Temporal artery temperature measurements in healthy infants, children, and adolescents. Clin Pediatr 2003;42:433-437.
19. Greenes DS, Fleisher G. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med 2001;155:376-381.
20. Graneto JW, Soglin DF. Maternal screening of childhood fever by palpation. Pediatr Emerg Care 1996;12:183-184.
21. Plaisance KI, Mackowiak PA. Antipyretic therapy: Physiologic rationale, diagnostic implications, and clinical consequences. Arch Intern Med 2000;160:449-456.
22. Aronoff DM, Neilson EG. Antipyretics: Mechanisms of action and clinical use in fever suppression. Am J Med 2001;111:304-315.
23. Macknin ML, Piedmonte M, Jacobs J, et al. Symptoms associated with infant teething: A prospective study. Pediatrics 2000;105(4 Pt 1):747-752.
24. Barlow BS, Kanellis MJ, Slayton RL. Tooth eruption symptoms: A survey of parents and health professionals. ASDC J Dent Child 2002;69:148-150, 123-144.
25. Wake M, Hesketh K, Lucas J. Teething and tooth eruption in infants: A cohort study. Pediatrics 2000;106:1374-1379.
26. Lloyd S. Teething in babies: Separating fact from fiction. Prof Care Mother Child 1996;6:155-156.
27. Cunha B. The clinical significance of fever patterns. Infect Dis Clin North Am 1996;10:33-44.
28. Simon HK. Current Concepts: Hyperthermia. N Engl J Med 1993;329: 483-487.
29. Stewart CE. Heat Emergencies. In: Stewart CE, ed. Environmental Emergencies. Baltimore: Williams and Wilkins; 1990:1-27.
30. Stewart CE. The spectrum of heat illness in children. Pediatr Emerg Med Rep 1999;5:175.
31. Gilat T, Shibolet S, Sohar E. Mechanism of heat stroke. J Tropi Med Hyg 1963;66:204-212.
32. Neymann CA, Osborne SL. Artificial fever. Am J Syphilis Neruol 1934; 18:34.
33. Attia M, Khogali M, El-Khatib G. Heat stroke: An upward shift of temperature regulation set point at an elevated body temperature. Int Arch Occup Environ Health 1983;53:9-17.
34. Johnson R. Management of pediatric fever without source. CAP-ACEP Lifeline: 07-08-2001, Accessed June 4, 2003. http://www.calacep.org/ lifeline/displaylifeline.html?ID=255.
35. Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: Have parental misconceptions about fever changed in 20 years? Pediatrics 2001;107: 1241-1246.
36. Schmitt BD. Fever phobia: Misconceptions of parents about fevers. Am J Dis Child 1980;134:176-181.
37. May A, Bauchner H. Fever phobia: The pediatrician’s contribution. Pediatrics 1992;90:851-854.
38. Poirier MP, Davis PH, Gonzalez-del Rey JA, et al. Pediatric emergency department nurses’ perspectives on fever in children. Pediatr Emerg Care 2000;16:9-12.
39. Duff GW. Is fever beneficial to the host: A clinical perspective. Yale J Biol Med 1986;59:125-130.
40. Kluger MJ. The adaptive value of fever. In: Mackowiak PA, ed. Fever: Basic Mechanisms and Management. New York: Raven Press; 1991:105-124.
41. Mackowiak PA. Assaulting a physiological response. Clin Infect Dis 1997;24:1214-1216.
42. Mackowiak PA. Fever: Blessing or curse? A unifying hypothesis. Ann Intern Med 1994;120:1037-1040.
43. Garrison FH. An Introduction to the History of Medicine: With Medical Chronology, Suggestions for Study and Bibliographic Data, 4th ed. Philadelphia: W. B. Saunders; 1929.
44. Kluger MJ, Kozak W, Conn CA. The adaptive value of fever. Infect Dis Clin North Am 1996;10:1-20.
45. Alexander HR, Sheppard BC, Jensen JC, et al. Treatment with recombinant tumor necrosis factor-alpha protects rats against lethality, hypotension, and hypothermia of gram negative sepsis. J Clin Invest 1991;88:34-39.
46. Evered LM. Does acetaminophen treat fever in children? Ann Emerg Med 2003;41:741-743.
47. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr OP Infect Dis 2002:15:241-245.
48. Doran T, DeAngelis C, Baumgardener R. Acetaminophen: More harm than good for chickenpox? J Pediatr 1989;114:1045.
49. Kushner I, Rzewnicki DL. The acute phase response. In: Mackowiak PA, ed. Fever: Basic Mechanisms and Management, 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1997:165-176.
50. Mackowiak PA. Physiological rationale for suppression of fever. Clin Infect Dis 2000;31 Suppl 5:S185-189.
51. Beisel WR, Morgan BB Jr, Bartelloni PJ. Symptomatic therapy in viral illness: A controlled study of effects on work performance. JAMA 1974;228: 581-584.
52. Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001;58: 445-452.
53. Mackowiak PA, Plaisance KI. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci 1998;856:214-223.
54. Treluyer J, Tonnelier S, d’Athis P. Antipyretic efficacy of an initial 30 mg/kg loading dose of acetaminophen versus a 15 mg/kg maintenance dose. Pediatrics 2001;108.
55. Walson PD, Galletta G, Chomilo F, et al. Comparison of multidose ibuprofen and acetaminophen therapy in febrile children. Am J Dis Child 1992; 146:626-632.
56. Wilson JT, Brown RD, Bocchini JA, Jr., et al. Efficacy, disposition, and pharmacodynamics of aspirin, acetaminophen, and choline salicylate in young febrile children. Ther Drug Monitor 1982;4:147-180.
57. Done AK. Treatment of fever in 1982: A review. Am J Med 1983;74:27-35.
58. Andersen AR. Parental perception and management of school-age children’s fevers. Nurse Pract May 1988;13:8-9, 12-18.
59. Aicardi J. Febrile convulsions. In: Aicardi J, ed. Epilepsy in Children, 2nd ed. New York, NY: Raven Press; 1994:253-275.
60. Rosman NP. Febrile convulsions. In: Mackowiak PA, ed. Fever: Basic Mechanisms and Management, 2nd ed: Lippincott-Raven Publishers; 1997:267-277.
61. Camfield PR, Camfield CS, Shapiro SH. The first febrile seizure: Antipyretic instruction plus either phenobarbital or placebo to prevent recurrence. J Pediatr 1980;97:16-21.
62. Uhari M, Rantala H, Vainionpaa L, et al. Effect of acetaminophen and of low intermittent doses of diazepam on prevention of recurrences of febrile seizures. J Pediatr 1995;126:991-995.
63. Schnaiderman D, Lahat E, Sheefer T. Antipyretic effectiveness of acetaminophen in febrile seizures: Ongoing prophylaxis versus sporadic usage. Eur J Pediatr 1993;152:747-749.
64. Van Stuijvenberg M, Derksen-Lubsen G, Steyerberg EW. Randomized controlled trial of ibuprofen syrup administered during febrile illnesses to prevent febrile seizure recurrences. Pediatrics 1998;102:E51.
65. Newman J. Evaluation of sponging to reduce body temperature in febrile children. CMAJ 1985;132:641-642.
66. McCarthy PL, Grundy GW, Spiesal SZ, et al. Bacteremia in children: An outpatient clinical review. Pediatrics 1976;57:861-868.
67. McGowan JE Jr, Bratton L, Klein JO, et al. Bacteremia in febrile children seen in a “walk-in” pediatric clinic. N Engl J Med 1973;288:1309-1312.
68. Teele DW, Pelton SI, Grant MJ. Bacteremia in febrile children under 2 years of age: Results of cultures of blood of 600 consecutive febrile children seen in a “walk-in” clinic. J Pediatr 1975;87:227-230.
69. Bonadio WA, Romine K, Gyuro J. Relationship of fever magnitude to rate of serious bacterial infections in neonates. J Pediatr 1990;116:733-735.
70. Yamamoto LT, Wigder HN, Fligner DJ. Relationship of bacteremia to antipyretic therapy in febrile children. Pediatr Emerg Care 1987;3:223-227.
71. Baker MD, Fosarelli PD, Carpenter RO. Childhood fever: Correlation of diagnosis with temperature response to acetaminophen. Pediatrics 987;80:315-318.
72. Torrey SB, Henretig F, Fleisher G, et al. Temperature response to antipyretic therapy in children: Relationship to occult bacteremia. Am J Emerg Med 1985;3:190-192.
73. Weisse ME, Miller G, Brien JH. Fever response to acetaminophen in viral vs. bacterial infections. Pediatr Infect Dis J 1987;6:1091-1094.
74. Baker RC, Tiller T, Bauscher JC. Severity of disease correlated with fever reduction in febrile infants. Pediatrics 1989;83:1016-1019.
75. Mazur LJ, Jones TM, Kozinetz CA. Temperature response to acetaminophen and risk of occult bacteremia: A case control study. J Pediatr 1989;115: 888-891.
76. Bonadio WA, Bellomo T, Brady W. Correlating changes in body temperature with infectious outcome in febrile children who receive acetaminophen. Clin Pediatr (Phila) 1993;32:343-346.
77. Mazur LJ, Kozinetz CA. Fever response to acetaminophen [letter]. Am J Emerg Med 1995;13:100-101.
78. Bass JW, Wittler RR, Weisse ME. Social smile and occult bacteremia. Pediatric Inf Dis J 1996;15:541.
79. Kelly MT, Walson PD, Edge JH, et al. Pharmacokinetics and pharmacodynamics of ibuprofen isomers and acetaminophen in febrile children. Clin Pharmacol Ther 1992;52:181-189.
80. Nahata MC, Powell DA, Durrell DE, et al. Efficacy of ibuprofen in pediatric patients with fever. Int J Clin Pharmacol Ther Toxicol 1992;30:94-96.
81. Kauffman RE, Sawyer LA, Scheinbaum ML. Antipyretic efficacy of ibuprofen vs acetaminophen. AJDC 1992;146:626-632.
82. Autret E, Breart G, Jonville AP, et al. Comparative efficacy and tolerance of ibuprofen syrup in children with pyrexia associated with infections diseases and treated with antibiotics. Eur J Clin Pharmacol 1994;46:197-201.
83. Wilson JT, Brown RD, Kearns GL. Single-dose, placebo-controlled comparative study of ibuprofen and acetaminophen antipyresis in children. J Pediatr 1991;119:803-811.
84. McIntyre J, Hull D. Comparing efficacy and tolerability of ibuprofen and paracetamol in fever. Arch Dis Child 1996;74:164-167.
85. Walson PD, Galletta G, Braden NJ, et al. Ibuprofen, acetaminophen, and placebo treatment of febrile children. Clin Pharmacol Ther 1989;46:9-17.
86. Marriott SC, Stephenson TJ, Hull D, et al. A dose ranging study of ibuprofen suspension as an antipyretic. Arch Dis Child 1991;66:1037-1041.
87. Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999;104:e39.
88. Hersh EV, Moore PA, Ross GL. Over the counter analgesics and antipyretics: A critical assessment. Clin Ther 2000;22:500-548.
89. Jack DB. One hundred years of aspirin. Lancet 1997;350:437-439.
90. Bruce-Chwatt LJ. Cinchona and its alkaloids: 350 years. Ann N Y Acad Sci 1998;856:214-223.
91. Cooper KE. Fever and Antipyresis: The Role of the Nervous System. Cambridge, Mass: Cambridge University Press; 1995.
92. Aronson SM. The miraculous willow tree. RI Med 1994;77:159-161.
93. Mackowiak PA. Brief history of antipyretic therapy. Clin Infect Dis 2000;31 Suppl 5:S154-156.
94. Vane JR. Aspirin and other anti-inflammatory drugs. Thorax 2000;55 (Suppl 2):S3-S9.
95. Starko KM, Ray CG, Dominguez LB. Reye’s syndrome and salicylate use. Pediatrics 1980;66:859-864.
96. Arrowsmith JB, Kennedy DL, Kuritsky JN, et al. National patterns of aspirin use and Reye syndrome reporting, United States, 1980-1985. Pediatrics 1987;79:858-863.
97. Belay ED, Bressee JS, Holman RE, et al. Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med 1999;340:1377-1382.
98. Spooner JB, Harvey JG. The history and usage of paracetamol. J Int Med Res 1976;4:1-6.
99. Simmons DL, Wagner D, Westover K. Non-steroidal anti-inflammatory drugs, acetaminophen, cyclooxygenase 2, and fever. Clin Infect Dis 2000; 31:S211-S218.
100. Anderson BJ, Holford NH, Woollard GA, et al. Paracetamol plasma and cerebrospinal fluid pharmacokinetics in children. Br J Clin Pharmacol 1998; 46:237-243.
101. Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the antipyretic activity of paracetamol (4-acetamidophenol). Nature 1972; 240:410-411.
102. Feldberg W, Gupta KP, Milton AS, et al. Effect of bacterial pyrogen and antipyretics on prostaglandin activity in cerebrospinal fluid of unanesthetized cats. Brit J Pharmacol 1972;46:550P-551P.
103. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol 1998;38:97-120.
104. Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 1995;44:1-10.
105. Schwartz JI, Chan CC, Mukhopadhyay S. Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin Pharmacol Ther 1999;65:653-660.
106. Houry D, Ernst A, Weiss S, et al. Ketorolac versus acetaminophen for treatment of acute fever in the emergency department. Southern Med J 1999;92: 1171-1173.
107. Vargas R, Maneatis T, Bynum L, et al. Evaluation of the antipyretic effect of ketorolac, acetaminophen, and placebo in endotoxin-induced fever. J Clin Pharmacol 1994;4:848-853.
108. Kearns GL, Leeder JS, Wasserman GS. Acetaminophen overdose with therapeutic intent [letter]. J Pediatr 1998;133(5).
109. Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994;272:953-956.
110. Rivera-Penera T, Gugig R, Davis J, et al. Outcome of acetaminophen overdose in pediatric patients and factors contributing to hepatotoxicity. J Pediatr 1997;130:300-304.
111. Schiodt FV, Rochling FA, Casey DL, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med 1997;272:1112-1117.
112. Heubi JE, Bien JP. Acetaminiphen use in children: More is not better. J Pediatr 1997;130:175-177.
113. Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: Hepatotoxicity after multiple doses in children. J Pediatr 1998;132:22-27.
114. Lesko SM, Mitchell AA. Renal function after short-term ibuprofen use in infants and children. Pediatrics 1997;100:954-957.
115. Lesko SM, Mitchell AA. An assessment of the safety of pediatric ibuprofen: A practitioner-based randomized clinical trial. JAMA 1995;273:929-933.
116. Stewart CE, Nixon RG, eds. Herbal Remedies Guidebook for EMS. Boston: Jones and Bartlett; 2003 (in press).
117. Mayoral CE, Marino RV, Rosenfeld W, et al. Alternating antipyretics: Is this an alternative? Pediatrics 2000;105:1009-1012.
118. Graneto J. Pediatrics, Fever. eMedicine Available at: www.emedicine.com/ emerg/topic377.htm. Accessed 6/2/2005.
119. Mofenson HC, McFee R, Caraccio T, et al. Combined antipyretic therapy: Another potential source of chronic acetaminophen toxicity. J Pediatr 1998;133:712-714.
120. Halpern SM, Fitzpatrick R, Volans GN. Ibuprofen toxicity: A review of adverse reactions and overdose. Adverse Drug React Toxicol Rev 1993; 12:107-128.
121. McIntyre J, Rubenstein RC, Gartner JC, et al. Acute flank pain and reversible renal dysfunction associated with non-steroidal anti-inflammatory drug use. Pediatrics 1993;92:459-460.
122. Bien JP, Heubi JE. Ibuprofen and/or acetaminophen: What price for “euthermia”? [letter-reply]. J Pediatr 1997;131:333.
123. Mackowiak PA. Antipyretic therapy’s future. Clin Infect Dis 2000;31 Suppl 5:S242-243.
124. Weiner JS, Khogali M. A physiological body cooling unit for treatment of heat stroke. Lancet 1980;1:507-508.
125. Steele RW, Tanaka PT, Lara RP, et al. Evaluation of sponging and of oral antipyretic therapy to reduce fever. J Pediatr 1970;77:824-829.
126. Schmitt BD. Fever in childhood. Pediatrics 1984;74(Suppl):929-936.
127. Axelrod P. External cooling in the management of fever. Clin Infect Dis 2000;31(Suppl 5):S224-S229.
128. Garrison RF. Acute poisoning from use of isopropyl alcohol in tepid sponging. JAMA 1953;152:317-318.
129. Senz EH. Coma in a child following use of isopropyl alcohol in sponging. J Pediatr 1958;53:322-323.
130. McFadden SW, Haddow JE. Coma produced by topical application of isopropanol. Pediatrics 1969;43:622-623.
131. Wise JR. Alcohol baths. N Engl J Med 1969;280:840.
132. Arditi M, Killner MS. Coma following the use of rubbing alcohol for fever control. Am J Dis Child 1987;141:237-238.
133. Stewart CE. Personal observation.
134. Aksoylar S, Aksit S, Caglayan S, et al. Evaluation of sponging and antipyretic medication to reduce body temperature in febrile children. Acta Paediatr Jpn 1997;39:215-217.
135. Agbolosu NB, Cuevas LE, Milligan P, et al. Efficacy of tepid sponging versus paracetamol in reducing temperature in febrile children. Ann Trop Paediatr 1997;17:283-288.
136. Hunter J. Study of antipyretic therapy in current use. Arch Dis Child 1973; 48:313-315.
137. Sharber J. The efficacy of tepid sponge bathing to reduce fever in young children. Am J Emerg Med 1997;15:188-192.
138. O’Donnell J, Axelrod P, Fisher C, et al. Use and effectiveness of hypothermia blankets for febrile patients in the intensive care unit. Clin Infect Dis 1997;24:1208-1213.
