Acute Ataxia and Gait Disturbances in the Emergency Department
December 1, 2022
Reprints
AUTHORS
Wan-Tsu W. Chang, MD, Associate Professor, Departments of Emergency Medicine and Neurology, Program in Trauma, University of Maryland School of Medicine, Baltimore
Christina M. Powell, DO, Department of Emergency Medicine, University of Maryland Medical Center, Baltimore
PEER REVIEWER
Catherine A. Marco, MD, FACEP, Professor, Department of Emergency Medicine, Penn State Health – Milton S. Hershey Medical Center, Penn State College of Medicine
EXECUTIVE SUMMARY
- Dizziness or unsteadiness is a common yet challenging presentation in the emergency department (ED). Assessment of the presenting complaints includes identifying whether the patient has ataxia or a gait disturbance.
- The main causes of ataxia can be separated into vestibular, sensory, cerebellar, and antalgic ataxia. Detailed physical exam findings in combination with risk factor assessment and presenting complaints narrow the decision tree. Patients can present atypically, and multiple causes of ataxia can exist in the same patient simultaneously.
- In general, drug-induced ataxia is reversible after discontinuing or lowering the dosage of the drug, but in some cases, symptoms can persist. The most commonly reported medications linked to drug-induced ataxia are antiepileptics, benzodiazepines, and antineoplastic agents.
- The goal of management of a patient presenting with ataxia or a gait disturbance is to prevent long-term neuronal damage, treat reversible causes, and improve debilitating symptoms. With prompt intervention, especially early in the disease course, most patients can regain most expected functions of daily living. As with many of these disease processes, the presentation can be atypical, and an interprofessional team should be called to evaluate each case promptly.
Introduction and Definitions
Ataxia and gait disturbances can signify a variety of conditions. The differential includes benign as well as life-threatening causes. An understanding of the pathophysiology and a thorough neurological exam are critical in making these distinctions.
Ataxia is defined as impaired coordination of voluntary muscle movement.1 It can involve the trunk, limb, or gait. Ataxia usually is caused by cerebellar dysfunction, including impaired vestibular or proprioceptive input to the cerebellum. Normal coordination, such as the ability to maintain balance, depends on the integration of cerebellar, vestibular, proprioceptive, visual, and musculoskeletal systems.1
Gait disturbances refer to abnormal patterns of walking, whether changes in fluency, smoothness, synchrony, or symmetry.2 While ataxia can be associated with an abnormal gait, most gait disorders are not due to impaired coordination. Gait disturbances can be caused by neurological conditions as well as orthopedic or medical conditions.3 Normal gait may seem like a simple activity, yet it also depends on the complex integration of multiple sensory and motor systems.
Relevance
In the emergency department (ED), attention must be focused on treatable and reversible etiologies of ataxia and gait disturbances. Ataxia can be seen in 30% to 60% of patients with posterior circulation stroke.4,5 However, studies have shown that misdiagnoses of posterior circulation strokes are frequent, with dizziness and difficulty walking more likely missed than focal weakness, vision changes, or neglect.6,7
Gait disorders contribute to reduced mobility and increased risk of falls, especially in the elderly. The National Hospital Ambulatory Medical Care Survey in 2018 described more than 26% of ED visits related to falls. About 20% of older adults require an assistive device to ambulate and one-third fall at least once a year.2 Reduced mobility leads to fall phobia, deconditioning, and further increased risk of falls as well as death from underlying conditions.8
Epidemiology
Approximately two-thirds of gait disorders are caused by neurological conditions.3 Advanced age, dementia, alcohol abuse, and the use of antiepileptics and neuroleptics also are risk factors. The prevalence of gait disorders increases as the population ages, with more than 60% affected in those older than 80 years of age.3
Etiology
Causes of ataxia include hereditary conditions such as spinocerebellar ataxias, acquired conditions such as toxic-metabolic disorders, as well as emergent conditions such as ischemic or hemorrhagic strokes.9,10 (See Table 1.) Hereditary ataxias are rare, although with advances in deoxribonucleic acid (DNA) testing they are diagnosed more frequently than they were previously.1 A detailed discussion regarding various types of hereditary ataxias is beyond the scope of this article.
Table 1. Causes of Ataxia |
|
Type |
Causes |
Genetic |
|
Toxic |
|
Metabolic |
|
Vascular |
|
Infectious |
|
Autoimmune |
|
Paraneoplastic Cerebellar Degeneration |
|
MELAS: mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; EBV: Epstein-Barr virus |
|
Gait disorders share only some etiologies with ataxia. Their classification extends beyond neurological conditions and includes musculoskeletal pathologies.2,8,11 (See Table 2.) Given the breadth of conditions causing ataxia and gait disturbances, this article will focus on treatable and reversible etiologies, with special emphasis on emergent or life-threatening causes.
Table 2. Causes and Characteristics of Gait Disorders |
|||
Etiology |
Gait Abnormality |
Characteristics |
|
Musculoskeletal |
|
Antalgic |
|
|
Myopathic |
|
|
Peripheral Nervous System |
|
Steppage |
|
|
Sensory ataxic |
|
|
Central Nervous System (Mid-Level) |
|
Hemiplegic (spastic) |
|
|
Paraplegic (spastic) |
|
|
|
Ataxic |
|
|
|
Parkinsonian (hypokinetic) |
|
|
|
Choreiform (dyskinetic) |
|
|
|
Dystonic (dyskinetic) |
|
|
Central Nervous System (High-Level) |
|
Freezing (akinetic) |
|
|
Apraxic |
|
|
Psychiatric |
|
Functional |
|
ALS: amyotrophic lateral sclerosis; PSP: progressive supranuclear palsy |
|||
Pathophysiology
Coordination, balance, and gait all require the integration of visual, vestibular, and proprioceptive information. Normal gait consists of locomotion, balance, and the ability to adapt to the environment.8
Cerebellum
The cerebellum is only 10% of the total weight and volume of the brain but contains half of the brain’s neurons.12 It receives input from the cerebral cortex as well as the spinal cord.1 (See Figure 1.) Lesions in the cerebellar vermis and the cerebellar hemispheres result in different ataxias. Midline cerebellar structures, including the vermis, modulate motor execution, eye movements, balance, and vestibular function; thus, injury causes truncal and gait ataxias, ocular findings, and vertigo. The cerebellar hemispheres integrate sensory input and are responsible for motor planning of complex tasks, thus, injury causes ipsilateral limb ataxia, dysdiadochokinesia, dysmetria, and scanning speech.1
Figure 1. Cerebellar Connections and Functions |
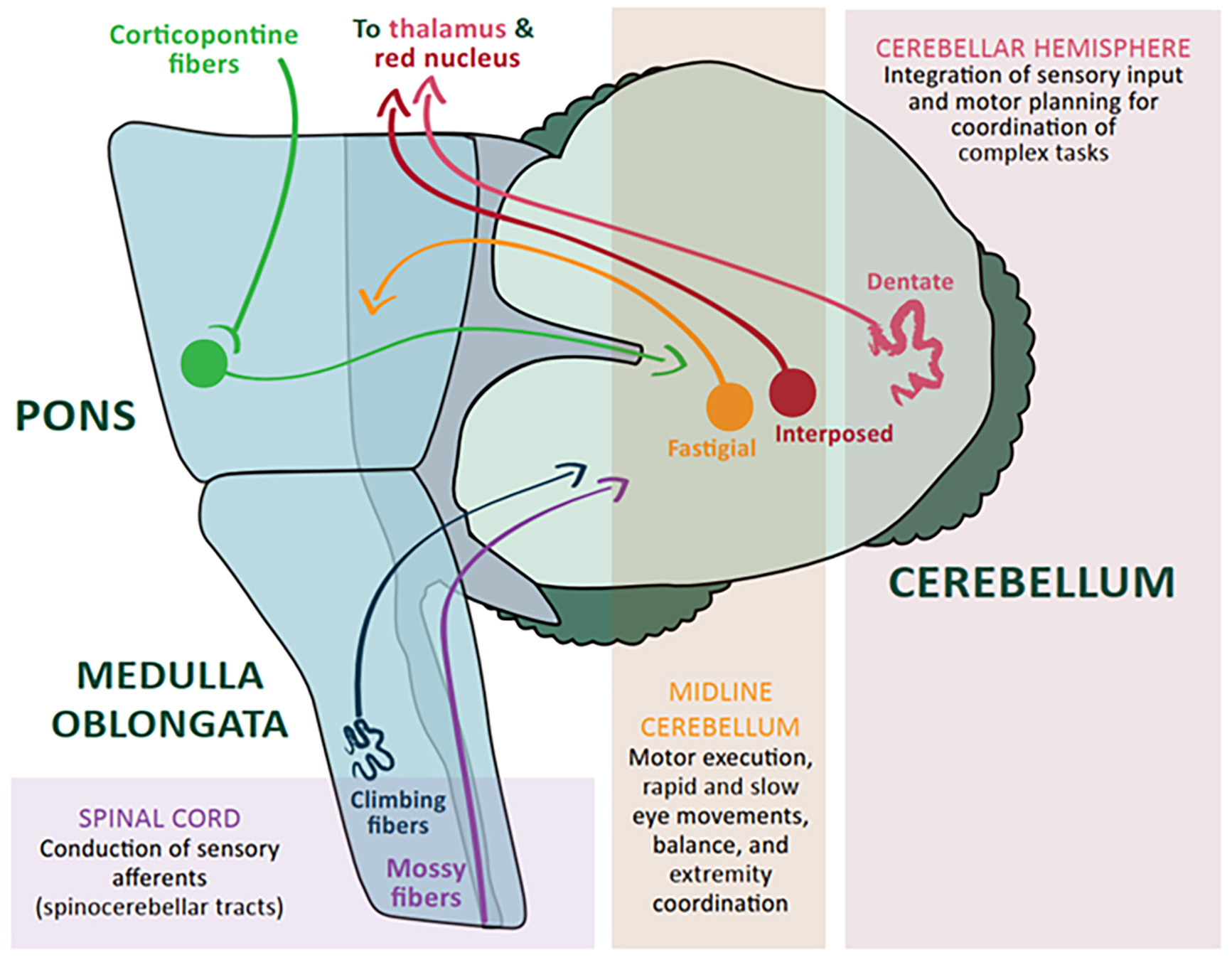 |
Courtesy of Christina M. Powell, DO. |
Cerebellar neurons are particularly susceptible to toxic-metabolic insults and consume one of the highest amounts of oxygen within the central nervous system.13 Four out of five types of cerebellar neurons are inhibitory in nature, using the neurotransmitter gamma-aminobutyric acid (GABA).12 Ethanol and several antiepileptic medications enhance GABA inhibition, thereby impairing motor coordination, while chronic ethanol use causes cerebellar vermian degeneration and atrophy, seen in more than 40% of alcoholics.14 Hypoxia, carbon monoxide poisoning, and hyperthermia cause Purkinje cell death and degeneration of cerebellar efferent pathways, resulting in ataxia as well as cortical, pyramidal, and extrapyramidal abnormalities.13
Nutritional inadequacies, in particular thiamine deficiency, cause neuronal dysfunction. Thiamine is critical in carbohydrate metabolism, synthesis of neurotransmitters, production of nucleic acids, and protection against oxidative stress.15 The cerebellar vermis is uniquely sensitive and most commonly damaged in thiamine deficiency. However, neurocognitive deficits also are seen, such as in Wernicke encephalopathy, due to disruption of neural pathways between the cerebellum and the frontal lobes.15
Vestibular System
Input to the vestibular system comes from the labyrinths, within which the specialized sensory cells detect acceleration and gravity.16 The vestibulocochlear nerve travels with the facial nerve through the internal auditory canal, then traverses the cerebellopontine angle and enters the brainstem at the pontomedullary junction. The vestibular fibers connect with the motor nuclei of the extraocular muscles to mediate the vestibulo-ocular reflex that stabilizes the eyes, while connections to the spinal cord via the vestibulospinal tracts stabilize the head and body.17 Direct connection with the cerebellum allows postural feedback based on vestibular input.
Vertigo, the illusion of movement of the body or the environment, indicates a disorder of the vestibular system.18 It often is accompanied by nausea, vomiting, and ataxia. Peripheral vestibular lesions affect the labyrinth or the vestibular division of the eighth cranial nerve, such as in benign paroxysmal positional vertigo (BPPV), Meniere’s disease, or vestibular neuritis. Central vestibular lesions affect the brainstem vestibular nuclei or their connections, such as in stroke or multiple sclerosis.16 Horizontal nystagmus is present with peripheral vertigo, usually unidirectional. If nystagmus is present with central vertigo, it can be horizontal, vertical, torsional, unidirectional, or multidirectional.
Proprioceptive Pathways
Proprioception, “the sense of self,” is the sense of the body’s position in space without the use of vision. Large, myelinated fibers carry this information via the dorsal column of the spinal cord to the brainstem, thalamus, and finally the sensory cortex of the parietal lobe. Dysfunctions in proprioception can occur from large fiber polyneuropathy, such as diabetic neuropathy, posterior root lesions, such as tabes dorsalis, and posterior column disease, such as multiple sclerosis and vitamin B12 deficiency. Patients with dysfunction in proprioception experience sensory ataxia. They may demonstrate a positive Romberg test where they lose their balance with their eyes closed because of the lack of visual compensation for their positional sense.
Clinical Features
Chief Complaint
Ataxia is a sign, not a diagnosis; therefore, patients likely will present with complaints of feeling “dizzy,” “off balance,” “uncoordinated,” or their “equilibrium being off.” They may describe needing to hold onto the railing or wall when going up or down stairs. Similarly, it is unlikely that a patient will present stating that they have a gait disturbance when their symptoms are early or mild. More often, they may present with “weakness,” “legs feeling heavy,” “trouble walking,” or observed by others to have an abnormal gait. It also is possible that the patient may not present for medical attention until they experience falls or injuries as result of their gait disturbance.
History
The time course or tempo of symptoms is essential in differentiating emergent causes of ataxia.1,10,19 (See Table 3.) An acute onset may be suggestive of stroke, infection, or toxic exposure, whereas an episodic nature may be consistent with a transient ischemic attack. Localizing factors associated with the presentation can lead toward an affected sensory pathway through the vestibular system vs. a more central localizing pathology in the setting of an acute stroke. Key associated symptoms can narrow the differential and may need collateral historian additional information. Common associated symptoms include slurred speech, abnormal eye movements, change in behavior, difficulty swallowing, increased fatigue, difficulty buttoning shirts, typing, or holding objects, or a history of dropping objects. Associated symptoms such as headache, nausea, vomiting, and dysarthria are concerning for posterior fossa pathology. Cognitive impairment and hallucinations are seen in Wernicke-Korsakoff syndrome, while urinary incontinence may reflect normal pressure hydrocephalus. To better understand the severity of symptoms, it is helpful to ask whether the patient can perform activities of daily living, such as getting dressed by themselves or getting to the bathroom at night. Gait disturbances due to sensory ataxias worsen in the dark.8 It also is important to assess the risk of falls by collateral historians to determine the patient’s safety in their living environment. The historian may not offer these details as part of their own history telling, and they may need prompting by the astute physician.
Table 3. Causes of Ataxia by Time Course of Symptoms |
|||
Acute (Hours to Days) |
Subacute (Weeks) |
Chronic (Months to Years) |
Episodic |
|
|
|
|
HIV: human immunodeficiency virus |
|||
Family History
Inherited forms of ataxia should be considered based on family history. Autosomal dominant ataxias typically present in the third or fourth decade of life compared to autosomal recessive ataxias, which present in childhood or young adulthood.19 Consider familial risk factors for diabetes, hypertension, and stroke for alternative causes, such as peripheral neuropathies or ischemic events.
Social History
Inquire about chronic or acute alcohol use, tobacco use, illicit drug use, or toxin exposures based on occupation, age of the home (such as with lead exposure), or other environmental hazards. For example, nicotine use can worsen limb ataxia and increase head nodding movements in some hereditary and degenerative ataxias.13
Medications
Pay special attention to medications and associated side effects, including vitamins and supplements, and over-the-counter and prescription medications. Ask about recent changes and who monitors therapeutic levels. Coordination with local or hospital pharmacists and online databases can help display recently filled medications and prescriptions to provide additional history if unable to be provided by the patient or collateral historian.
Physical Exam
Examination of a patient with ataxia or gait disturbance is not limited to the neurological assessment. Vital signs may point to hypovolemia and explain orthostatic symptoms. External signs of head trauma heighten the concern for an intracranial injury, such as subdural or epidural hematoma. Patients with atrial fibrillation not on anticoagulation have an increased risk of stroke. Look for rashes, stigmata of liver disease, lower extremity edema, and deformity and wounds of the feet for clues to the etiology of the patient’s symptoms. Walking patients at least 12 feet can reveal associated symptoms, such as lightheadedness, shortness of breath, and extremity fatigue at onset or after extended use. If there are no safety concerns, avoid aiding the patient too early in the exam or abnormal findings may remain hidden. Keep in mind the patient’s baseline ambulatory status may need to be provided by a family member or caregiver.
Mental Status. Report of ataxia or gait disturbance with subsequent decline in level of consciousness is highly concerning for an acute intracranial process, such as stroke, hemorrhage, or mass with cerebral herniation. While the most prominent sign of cerebellar dysfunction is incoordination, cognitive dysfunctions such as memory problems, spatial disorientation, or personality changes may be seen.20 Patients with cerebellar dysfunction also can have slowing of speech with scanning speech, also called scanning dysarthria, where words are broken into syllables with unusual stressing of plosive consonants.1 Toxin and metabolic toxidromes also can lead to mental status changes.
Cranial Nerves. Examination of the cranial nerves is critical given the proximity and relation between the cerebellum and the brainstem. Fundoscopic exam in patients presenting with headache or comatose state may reveal papilledema indicating elevated intracranial pressure. Vision loss or internuclear ophthalmoplegia with ataxia may signify demyelinating diseases, such as multiple sclerosis, that may warrant immediate consultation.
Evaluate ocular movements by observing the smoothness of gaze and any restrictions or dysconjugate movements. Have the patient change focus quickly between the examiner’s finger and nose to evaluate corrective saccade.20 Look for nystagmus of the eyes in the primary position as well as with gaze. In patients who present with episodic positional vertigo, perform the Dix-Hallpike maneuver to diagnose benign paroxysmal positional vertigo. In patients who present with continuous vertigo, perform the head impulse test, nystagmus, test of skew (HINTS) exam with the designation “HINTS central” or “HINTS peripheral” instead of the more commonly used “HINTS negative” or “HINTS positive,” which is ambiguous in determining the affected location.21 Cerebellar dysfunction can be associated with abnormal visual pursuit, corrective saccades, and upbeat or downbeat nystagmus, depending on the location of the lesion. Asymmetric horizontal gaze-evoked nystagmus may be caused by an ipsilateral cerebellar or vestibular lesion. However, nystagmus in peripheral vertigo can be inhibited by visual fixation.20
Facial or tongue fasciculations as well as tongue atrophy may be seen in subtypes of spinocerebellar ataxia. Tongue fasciculations among other signs also can be present in alcohol withdrawal states. Especially in the elderly population, acute hearing loss or a change from baseline can indicate an inner ear or vestibular etiology.
Motor. The strength exam helps determine whether muscle weakness is contributing to ataxia. Start by having the patient hold out both arms supinated with palms toward the ceiling. Unilateral pronation or drift suggests weakness or a sensory deficit, most concerning for an acute infarction. Next, isolate each muscle group and look for symmetric vs. asymmetric and proximal vs. distal weakness. Have the patient attempt to stand on their toes and heels. Myopathy is characterized by symmetric proximal muscle weakness, while neuropathy is associated with distal muscle weakness. Hypotonia can be seen in certain hereditary ataxia syndromes, while muscle hypertonia, or spasticity, can follow an upper motor neuron disease.
Truncal ataxia involves incoordination of the muscles close to the trunk, including torso, hips, and shoulders. The patient will present with a wide-based gait or have difficulty staying upright when seated. These patients may appear unstable or worse with their arms stretched out in front of their body when sitting. Titubation also can be noted, which refers to an essential tremor involving uncontrollable, rhythmic shaking, most commonly in the head.2
Limb ataxia can involve one limb, both limbs, or all four extremities. Patients can present complaining of difficulty buttoning their shirt, picking up objects, and other functional difficulties.1 Extremity tremor also can be a presenting symptom.
Sensory. A full sensory exam includes light touch, pin prick, temperature, vibration, and proprioception. Pay particular attention to unilateral abnormalities. The pattern of impairment, including a sensory level, helps differentiate between neuropathy and myelopathy. The Romberg test removes the visual component of balance, allowing evaluation of the dorsal column of the spinal cord. A positive or abnormal Romberg test demonstrates increased unsteadiness or loss of balance with the eyes closed. Loss of balance can be defined as the increased swaying of the body, foot movement in the direction of the fall, or actual falling.22 The physician administering the exam should stand close to the patient to prevent any possible injury. Pseudoathetosis (random finger movements observed when a patient’s hand is outstretched when their eyes are closed) also may be seen in proprioceptive disease.1
Reflexes. Deep tendon reflexes should be compared from one side of the body to the other and between upper and lower extremities. Hyperreflexia is associated with upper motor neuron lesions; however, these develop days to weeks after the injury. Clonus, or an involuntary and rhythmic muscle contraction, is caused by lesions in descending motor neurons. It can be induced by stretching the tendon in the ankle, patella, triceps, wrist, jaw, or biceps. Clonus can indicate a central etiology to the patient’s presentation.23 Conversely, hyporeflexia is seen in peripheral neuropathy, although significant myopathies may result in the muscle being too weak to contract. The Babinski or the extensor plantar reflex normally causes down-going or plantar flexion of the toes. Up-going or extension of the great toe and fanning of the other toes suggests damage to the corticospinal tract in adults but can be considered normal in infants up to 24 months of age.24
Similar to the Babinski reflex of the foot, the Hoffmann sign suggests an upper motor neuron lesion affecting the hands and can provide evidence for cervical cord compression.25
The Hoffman sign is an involuntary flexion movement of the thumb or index finger when the physician flicks the fingernail of the middle finger down. While presence of the Hoffman sign can help as a screening tool, it is not reliable as a stand-alone predictor of spinal cord compression.
Coordination. Tests of coordination include finger-to-nose, heel-knee-shin, and rapid alternating movements. Observe the speed and smoothness at which patients perform these tests. Notice any asymmetry in the movements as concerns for a focal finding. Cerebellar ataxia may be associated with dysmetria, dysdiadochokinesia, and intention and postural tremors.9 Truncal ataxia worsens with outstretched arms and standing. The patient may need help sitting upright unassisted, since they may fall toward one side or another. Intention or action tremor can be observed during finger-to-nose testing, especially as they get closer to the target and with repetition.9
Gait and Stance. Perhaps the most important neurological assessment in patients with ataxia and gait disturbances is to ambulate the patient and assess their gait. A lot can be learned just by watching the patient walk around the ED or headed toward the bathroom seemingly unobserved. Observe how they get out of the chair or stretcher. Ask the patient to walk across the room and turn back. If the patient completes this safely, then ask them to walk heel-to-heel, or tandem walk, which may reveal abnormalities that are not obvious. Observe their posture, balance, and stride. When walking normally, 60% of the gait cycle is the stance phase with 10% of that time with bipedal support. The remaining 40% is the swing phase where the foot is in the air.2 Healthy individuals can stand naturally with feet spread less than 12 cm apart or in tandem for more than 30 seconds and require only one to two steps to make a 180-degree turn.1,19
Abnormal gait may be the result of cerebellar, vestibular, or sensory dysfunction. Patients with cerebellar dysfunction have a wide-based stance and gait with staggering and irregular steps. This may be more pronounced when the patient turns or stops abruptly.9 Vestibular pathology causes patients to veer to the ipsilateral side when attempting to walk in a straight line. Gait disturbance with sensory ataxia worsens in the dark because of impaired visual compensation. Patients need to carefully watch the ground to improve their stability.
Antalgic gaits typically are due to pain and discomfort and are observed to be limping on exam. A buckling gait, such as the knees giving way with ambulation, can be seen in patients with myoclonus of the lower extremities or muscle weakness.26 Evaluate for the underlying physical injury with the appropriate musculoskeletal examination. An observed buckling gait, in the presence of normal quadriceps strength on physical exam, would be evidence toward a functional gait abnormality. A commonly observed inconsistency in patients with functional gait disorder resembling ataxia is that balance control is much better than what is perceived by the patient. The patient may use support of chairs, doorposts, or walls on exam but will not fall when the support structure is absent.26 Table 2 summarizes classic characteristics of various gait abnormalities.
Diagnostic Studies
Obtaining laboratory or imaging studies is pursued on a case-by-case basis. The goal is to screen for treatable and reversible causes of acute presentations. Table 4 describes some of the basic laboratory tests ordered to evaluate for toxic, metabolic, inflammatory, autoimmune, nutritional, endocrine, neoplastic, or infectious causes of ataxia and gait disturbance. Most patients have a basic laboratory work-up, including complete blood count (CBC) with peripheral smear, complete metabolic panel (CMP) including calcium and magnesium, coagulation studies, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), thyroid-stimulating hormone (TSH)/Free T4, and lactate (for mitochondrial disorders).
Table 4. Basic Laboratory Testing for Evaluation of Ataxia |
||||
Toxic and Metabolic |
Inflammatory |
Nutritional |
Neoplastic |
Infectious |
|
|
|
|
|
ANA: antinuclear antibodies; anti-TPO: anti-thyroid peroxidase; SSA: Sjögren syndrome A; SSB: Sjögren syndrome B; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; HbA1c: hemoglobin A1c; TSH: thyroid-stimulating hormone; CBC: complete blood count; SPEP: serum protein electrophoresis; RPR: rapid plasma reagin; HTLV: human T-lymphotropic virus; HIV: human immunodeficiency virus |
||||
Laboratories
In selective patients presenting with altered mental status, bizarre behavior, or focal findings, consider lumbar puncture for opening pressure, cerebral spinal fluid (CSF) cell count, protein, glucose, cultures, viral panels, oligoclonal bands, or myelin basic protein. In a patient with suspected malignancy, or underlying risk factors for malignancy, consider paraneoplastic panel cytology, lactate, and cerebellar autoantibodies. Many special CSF tests, auto-antibody tests, and other serum genetic tests will be ordered in combination with neurology consultation, admission, or outpatient evaluation. Assess coagulation studies and head imaging prior to lumbar puncture because of concerns for thrombocytopenia and herniation syndromes, respectively.
Consider serum human immunodeficiency virus (HIV), rapid plasma reagin (RPR), and Lyme disease titers in patients with risk factors or prior untreated diagnoses. The reach of the COVID-19 pandemic extends into the topic of ataxia as well, and viral studies should be ordered. In one of the first reports on neurological findings during the COVID-19 outbreak in Wuhan, China, neurological manifestations were present in 78/214 patients (36.4%), and ataxia was most common in the severely affected patients.27 In a recent literature review, 51 cases of myoclonus and ataxia associated with COVID-19 were identified, and more than 41% of cases had ataxia alone.28 Three cases were asymptomatic for COVID-19 and presented with ataxia and neurologic symptoms alone.
If Wernicke encephalopathy is suspected, immediate thiamine replacement takes precedence over laboratory testing. Ordering a thiamine level can facilitate definitive diagnosis for inpatient management. Consider carboxyhemoglobin level if there is suspicion of carbon monoxide (CO) poisoning either from occupational or environmental exposures. If drug-induced ataxia is suspected, therapeutic drug levels or levels for drugs of intoxication should be ordered accordingly. A blood alcohol level of 150 mg/dL to 300 mg/dL will affect coordination and balance leading to ataxia.1 A sample list of drugs associated with cerebellar disease and ataxia is listed in Table 5.
Table 5. Drugs and Toxins Associated with Ataxia |
|
Additional Studies and Imaging
Consider an electrocardiogram (ECG) to assess for any underlying pathology, including arrhythmia, atrial fibrillation, QTC prolongation, wide QRS, or congenital abnormalities. Cardiac disease can present in atypical ways, manifesting with neurologic sequelae as blood flow to the neurons is disrupted.
Brain imaging for emergent causes of ataxia and gait disturbance includes a computed tomographic (CT) scan without contrast to rapidly evaluate for intracranial hemorrhage. Acute focal neurologic findings may be evaluated initially in accordance with the hospital’s stroke protocol. This may include emergent neurology consultation, implementing the National Institutes of Health Stroke Scale (NIHSS), and CT angiography of the head and neck with perfusion studies to assess for ischemic changes, vascular stenosis, or embolic pathology. Keep in mind that the NIHSS is weighted toward the anterior circulation. Patients presenting with acute ataxia concerning for posterior circulation involvement can present with a low NIHSS with disabling deficits.29 Anticoagulation and tissue plasminogen activator (tPA) will be discussed by the primary team as indicated. An initial CT brain without contrast can demonstrate any acute hemorrhage, large masses, gross atrophy, or hydrocephalus, while contrasted studies aid investigation for intracranial abscesses or infectious infiltration.
Magnetic resonance imaging (MRI) of the brain can visualize underlying ischemic, infectious, or anatomic pathology, likely in concert with neurology and interventional radiology consultation either in the inpatient or outpatient setting, depending on clinical presentation. Brain MRI with diffusion-weighted imaging (DWI) represents the gold standard test for cerebellar infarction since it can visualize both poor perfusion and secondary signs of tissue damage.30 MRI of the spinal cord is the gold standard for myelopathy, cord compression, or epidural abscess. CT myelogram is an alternative imaging choice if the patient is unable to undergo magnetic imaging.
Differential Diagnosis
When a patient presents with “dizziness” or “weakness,” the differential can be overwhelming. The previous review in Table 3 divides causes of ataxia by time course; this can be a helpful starting point, keeping in mind atypical presentations.
Alternatively, the differential diagnostic list can be divided by the type of ataxia encountered. Main causes can be separated into vestibular, sensory, cerebellar, and antalgic ataxias. Detailed physical exam findings in combination with risk factor assessment narrow the decision tree. Nutritional deficiencies and toxic exposures, including polypharmacy, are explained separately since their pathophysiology can affect multiple pathways simultaneously. Table 6 summarizes the differential diagnoses by ataxia type.
Table 6. Differential Diagnosis Summary Table by Ataxia Type |
Vestibular
Sensory
Cerebellar
Other
|
CIDP: chronic inflammatory demyelinating polyneuropathy; CNS: central nervous system; |
Vestibular Ataxia
Ataxia caused by vestibular disease may present with the complaint of falling off toward one side, veering toward a side while ambulating, or symptoms associated with change in position. The presence of vertigo, tinnitus, and nystagmus on exam indicate a more vestibular cause.
Peripheral Causes. Benign proximal positional vertigo is a common episodic cause of ataxia, mainly due to dizziness elicted by changes in head position. Vestibular neuritis classically presents with vertigo, nausea, and gait imbalance. It is associated with a preceding or accompanying viral infection; however, despite common teaching, viral infection is reported to be absent in 50% of the cases.31 The addition of unilateral hearing loss increases the likelihood of labyrinthitis as the diagnosis. Meniere’s disease is described as episodic symptoms of vertigo, tinnitus, and hearing loss, and symptoms typically last less than 12 hours.31 Keep in mind that patients presenting with a peripheral vestibular cause may have a normal physical exam, especially if the symptoms are episodic.
Central Causes. Continuous symptoms are more often associated with a central cause, along with truncal instability, an unsteady gait, dysarthria, and other focal neurological symptoms. Seventy-five percent of posterior fossa strokes present with the complaint of dizziness or a sensation of falling toward one side.30 Posterior fossa strokes are less than 1% to 4% of total strokes but carry almost double the mortality of the more common cerebral strokes.30 The HINTS was 100% accurate in differentiating neuritis from stroke when completed by neurotologists, outperforming MRI (88% sensitive when done within the first 48 hours).32 It should be noted, however, that the HINTS test requires training both in performing and interpreting the test. Other differentials include vertebral artery dissection or space occupying lesion. While less common, there have been reports of intermittent ataxia with associated hearing loss and vertigo in patients presenting with vertebral artery dissection.33 Forty-five percent of patients with vestibular schwannomas, benign slow-growing tumors, presented with ataxia as one of their complaints.34 If left untreated, patients can have long-term sequelae of increased intracranial pressure with worsening symptoms.
Sensory Ataxia
Impairment along the spinocerebellar tract may cause sensory loss; consider posterior spinal cord syndrome, tertiary syphilis, Friedreich ataxia (staggering gait and frequent falls), acquired sensory ataxias related to ataxic polyneuropathies [e.g., paraneoplastic sensory neuropathy], SjÖgren syndrome, diabetes mellitus, vitamin B6 toxicity, vitamin B12 or E deficiency, and Miller-Fisher syndrome.
Hydrocephalus can cause compression of the corona radiata, leading to impaired coordination of voluntary motor control of the limbs with difficulty modulating sensory information from the body. Patients can be seen walking with a high-stepping gait due to associated motor weakness, or with a feet-slapping gait where sound waves are used as sensory feedback. Consider normal pressure hydrocephalus in the elderly population.
There are a few autoimmune conditions that are treatable, for example chronic inflammatory demyelinating polyneuropathy (CIDP) or Hashimoto thyroiditis, where the imbalance and gait abnormality are secondary to weakness plus proprioceptive impairment.35
Cerebellar Ataxia
Cerebellar infarction demonstrates ipsilateral physical exam findings, where the lateralizing findings localize to the same side as the lesion. Extremity tremor also can be a presenting symptom, lateralized to the side of the cerebellum affected. When confronted with a patient oscillating in a chair from side to side, consider a midline vermis lesion. In any ataxic patient with a lateralizing finding, consider acute cerebellar infarction, hemorrhage, or neoplastic process.
Spontaneous cerebellar hemorrhages are less common than traumatic hematomas but still are present as etiologies.36 While the most common etiology of cerebellar hemorrhage is hypertension, thrombocytopenia and other bleeding diatheses are well-recognized predisposing risk factors. Diseases that damage the spinal cord and peripheral nerves, such as epidural abscesses, cord compression, neoplastic lesions, or transverse myelitis, can cause limb ataxia, as the connection to the cerebellum is disrupted.37,38
Antalgic Ataxia
First, consider whether a gait disturbance is caused by a primary neurologic or metabolic cause. Afterward, one can consider antalgic gait patterns (due to pain), buckling gait (due to weakness), or waddling gait (due to pelvic girdle weakness) due to muscular involvement from injury or strain.
Functional gait disorders are common in the community setting and can be equally disabling for a patient’s ability to complete their daily activities. Functional gait disorder is preferred over the term psychogenic gait disorder, since it is more descriptive and does not carry a negative connotation.39 Both functional gait disorders and organic gait disorders can present with bizarre and atypical movements, and the presence of a functional gait disorder does not rule out an underlying organic cause.
Acquired Ataxia Associated with Nutritional Deficiencies
Individuals at risk for acquired ataxia secondary to nutritional deficiencies in developed countries include the poor and homeless, the elderly, patients on prolonged or inadequate parenteral nutrition, individuals with dieting constraints or eating disorders, chronic alcoholics, and individuals with past medical history significant for pernicious anemia, sprue, celiac disease, or inflammatory bowel disease and past surgical history of bariatric surgery.40 In these conditions, the neurologic exam will be emphasized, with one or more focused findings.
Thiamine (Vitamin B1) Deficiency. Thiamine deficiency associated with alcoholism is more frequently observed in males, whereas thiamine deficiency stemming from gastric–bariatric surgery is more common in females.41 Gait ataxia associated with findings of ocular abnormalities, such as horizontal and vertical nystagmus, ophthalmoparesis (weakness of one or more extraocular muscles), and/or conjugate gaze palsy would indicate late-stage thiamine deficiency. Wernicke encephalopathy is described as abnormal eye movement, gait and trunk ataxia, and altered mental status.13 Progression to Korsakoff syndrome will be evidenced by the previously mentioned findings plus amnesia and confabulation.
Folic Acid (Vitamin B9) Deficiency. Cerebral folate deficiency can present with progressive ataxia as well as seizures, developmental delay, and movement disorders. Folate occurs in higher concentrations in the CSF than in plasma, and enters the CSF against a concentration gradient. Many drugs are methylated during normal detoxification and can lead to cerebral folate deficiency, such as long-term L-dopa/carbidopa therapy, certain antiepilectics, methotrexate, and trimethoprim.42
Other B-Group Vitamin Deficiencies/Excess. Vitamin B12 deficiency, usually due to lack of intrinsic factor in atrophic gastritis or a vegan diet, induces sensory ataxia due to degeneration of the dorsal column and pyramidal tract. This occurs over months to several years. Deficient vitamin B12 can be seen in cases of neuropathy, suspected subacute combined degeneration of the spinal cord, or elderly patients with cognitive difficulties and gait problems.2 Vitamin B6 (pyridoxine) dosing exceeding 50 mg/d to 100 mg/d may induce neuropathy leading to sensory ataxia.1
Vitamin E Deficiency. Acquired vitamin E deficiency occurs due to underlying malabsorption syndromes, such as celiac disease, cystic fibrosis, eating disorder, or a surgical history of bowel resection. Vitamin E levels are considered deficient when they are below 2 mg/L in serum.43
Copper Deficiency. Acquired copper deficiency represents a rare cause of progressive myelopathy that can present with sensory ataxia and a spastic gait.44
Metabolic and Toxin Exposures
In general, drug-induced ataxia is reversible after discontinuing or lowering the dosage of the drug, but in some cases symptoms can persist. Table 5 lists commonly used drugs that are evidenced to cause acquired ataxia. The most commonly reported are antiepileptic drugs, benzodiazepines, and antineoplastic drugs. Interestingly, patients who chronically take one of these medications may develop acute cerebellar deficits without a change in their medication dosage.13
Antiepileptics. Phenytoin toxicity is seen with chronic use. Patients prescribed a lower dose of phenytoin tend to develop nystagmus and truncal ataxia, whereas appendicular ataxia becomes more prominent at higher doses.13 Patients on phenytoin at a therapeutic level have baseline nystagmus, and the finding increases as the serum level exceeds 20 mg/mL.13
Lithium toxicity can present with gait ataxia, dysarthria, and tremor from cerebellar disease.13 Carbamazepine’s neurologic findings also are dose-dependent. Free drug level can be elevated even if total serum concentration is therapeutic. The development of ataxia at increasing doses may be recoverable with cessation of treatment, but some patients may experience permanent cerebellar impairment.13 Other antiepileptics frequently used, such as talampanel, oxcarbazepine, and lamotrigine, have the highest risk of inducing cerebellar ataxia, with the number needed to harm of < 10.45
Benzodiazepines. Benzodiazepines, barbiturates, and other sedatives lead to excessive sedation prior to developing ataxia. The classic presentation of benzodiazepine overuse is central nervous system depression, including slurred speech, ataxia, and altered mental status. Ataxia is the most common sign of benzodiazepine toxicity in children, occurring in the majority of pediatric patients.46
Antineoplastic and Immuno-suppressive Medications. Many medicines, such as 5-fluorouracil, methotrexate, cytarabine, cisplatin, oxaliplatin, paclitaxel, capecitabine, and epothilone D, can be associated with neurologic side effects, particularly ataxia with high doses.13 Ataxia associated with cytarabine appears two to four days after the first dose and has been shown to resolve several weeks after cessation.45 Transplant medications, including tacrolimus and cyclosporin, are cacineurin inhibitors that have been shown to cause mild and transient ataxia. Advanced patient age, liver or kidney dysfunction, and polypharmacy were predisposing factors for the development of cerebellar toxicity.
Miscellaneous Medications. Bismuth, typically prescribed for gastrointestinal disorder, can induce a neurologic syndrome presenting with tremor and gait ataxia, altered mental status, myoclonus, and seizures. The use of amiodarone, among other antiarrhythmics, has many unfavorable side effects, including cerebellar toxicity. A pancerebellar syndrome of nystagmus, dysmetria, titubation, and ataxia of gait and stance has been evidenced in the literature.45 Symptoms were dose dependent. Similarly, procainamide and propafenone also can induce ataxia at supratherapeutic doses.
Nicotine. Chronic exposure to nicotine causes a decrease in the number of Purkinje cells in the cerebellar vermis.13 In patients with a past medical history of spinocerebellar ataxia or multiple system atrophy, nicotine use through cigarette smoking can worsen limb ataxia, and one may observe increasing head nodding movements.
Illicit Drug Use. Phenylcyclohexyl piperidine (PCP), cocaine, and heroin can lead to cerebellar disease due to damaged Purkinje cells. PCP overdose can present with gait ataxia, tremor, and nystagmus. Cocaine use can cause both ischemic and hemorrhagic stroke due to underlying hypertension, embolism, vasospasm, and vasculitis.13 Heroin overdose can clinically present as ataxia, dysarthria, and dementia, with associated lethargy, inattention, and forgetfulness.
Environmental Toxins. Heavy metal poisoning, such as mercury, copper, manganese, and iron, leads to neuronal cell death, with presenting symptoms of ataxia, cognitive dysfunction, or induced parkinsonism.47 Solvents and pesticide exposures, including arsenic, benzene, carbon disulfide, and lead, can lead to similar presentations. Consider the age of the home and other environmental hazards as possible precipitants.
Management
As with all patients presenting to the ED, immediate stabilization and resuscitative efforts take precedence, including attention to circulation, airway, and breathing. Since the Purkinje cells in the cerebellum are the most vulnerable to hypoxic insult, ensure adequate oxygenation and circulatory support for these patients. Treat vital sign abnormalities as appropriate. Purkinje cells are vulnerable to extreme temperature change. Treat hyperthermia with antipyretics, ice packs, cooling fans, and cooled saline to reach normothermia.
Initiate and follow appropriate protocols for ischemic stroke and large vessel occlusions, including blood pressure management, obtaining appropriate laboratory and imaging studies, and considering tissue plasminogen activator (tPA). Appropriate consultation with neurosurgery, neurology, and/or interventional radiology should be assessed based on the clinical presentation and imaging findings. Reactive cerebral edema following cerebellar infarct typically worsens over three to four days and can lead to subsequent herniation; therefore, these patients need appropriate disposition to a neurologic intensive care service.30
In the setting of cerebellar hemorrhage, reverse coagulopathy based on the patient’s medication list, clinical scenario, and abnormal coagulation studies. Complete serial neurologic exams and repeat head imaging studies in accordance with neurosurgical consultation to assess hemorrhage progression. Antiepileptics should be initiated, since meningeal irritation from blood products can cause epileptic activity. Additional imaging studies, including angiography or Doppler ultrasound, may be indicated for vascular pathology or spinal cord pathology. Vascular stenosis or dissection should lead to appropriate surgical consultation because endarterectomy or stenting may be indicated. Monitor the patient closely for signs of increasing intracranial pressure or changing neurologic deficits, and consider head of the bed elevation, mannitol, hypertonic saline, or hyperventilation in the acute setting if the patient deteriorates.
Apply 100% oxygen via non-rebreather mask for patients presenting with suspected CO poisoning. Consult hyperbarics if located at your facility or consider transfer if the COHb level is elevated.
Lumbar puncture may be indicated for diagnostic or therapeutic effect in the setting of idiopathic intracranial hypertension, normal pressure hydrocephalus, or if additional studies are needed for evaluation of meningitis, encephalitis, or cerebritis. Treat underlying infectious etiology with broad-coverage antibiotics with CNS penetration and/or antivirals until cultures result.
After initial evaluation with CT imaging, MRI brain or spinal cord may be indicated. For MRI findings of acute cord compression or other spinal lesion, consult the appropriate neurosurgical service for surgical management, along with IV antibiotics with CNS penetration if abscess is suspected. Patients with MRI findings but no acute weakness or focal neurologic deficit can be seen in an outpatient setting. Patients without imaging findings but with persistent symptoms may need admission for serial neurologic exams and observation.
Review current and recently discontinued or changed medications. Consult pharmacology if drug-drug interactions in the setting of polypharmacy or new renal or liver dysfunction are noted. If a patient is taking more than one medication evidenced to cause drug-induced ataxia, the drug with the highest likelihood of being the culprit should be stopped first (if possible), and the effect on the symptoms should be evaluated clinically.45
If the serum albumin level is low, certain albumin-binding medications, such as phenytoin, may need to be corrected. Urgent admission and rapid treatment of patients with supratherapeutic antiepileptics is necessary to avoid possible long-term neurologic deficits.13 Most drug-induced presentations are reversible; however, some antineoplastic medications and lithium have been shown to demonstrate persistent symptoms.
Consider medications for symptomatic control to treat associated complaints of vertigo, nausea, vomiting, or headache. If a peripheral vestibular cause is suspected, management may involve antiemetics (promethazine, metoclopramide), antihistamines (diphenhydramine, meclizine), and benzodiazepines (diazepam, lorazepam). Completing the Epley maneuver at the bedside can provide your patient immediate relief. Consultation with the appropriate service, such as otolaryngology, may be indicated in either the inpatient or outpatient setting, since vestibular schwannomas can have worsening symptoms with increased intracranial pressure without treatment or resection.
Additional Aspects
Initial assessment of life-threatening and permanently disabling causes of ataxia takes precedence. The differential for gait instability and ataxia is vast, and many diagnoses may occur in the outpatient setting. Neurologists should be consulted for guidance and further evaluation. Proper documentation of early diagnosis, early imaging, and early consultation can help prevent unnecessary medical or legal costs while working up a patient presenting with acute ataxia.
A pharmacist can perform medication reconciliation to see if any drugs are known to cause ataxia or gait abnormalities. The pharmacist can verify the dosing and counsel the patient on the proper administration, monitoring drug levels, adverse effects, and any drug-drug interactions.
Patient education is essential for secondary prevention of many of the disease processes discussed. Smoking cessation and proper treatment and evaluation of underlying comorbid conditions, such as atrial fibrillation, diabetes, and hypertension, should be advised. The emergency physician has the unique opportunity for patient education in an acute setting and ensuring proper outpatient and primary doctor follow-up, outpatient referral, proper medication compliance, and lifestyle modifications.
Disposition
Patient disposition depends on the acuity of the patient presentation, clinical suspicion of diagnosis, and time course of the presentation. Patients with unstable vital signs, clinically worsening symptoms, or acute deficits need admission to the hospital for monitoring. Findings of acute cerebellar hemorrhage or infarction will need admission to a neurosurgical critical care service for frequent neurologic reassessments and/or surgical intervention. Subacute or chronic presentations can be admitted to a medical floor for risk stratification and blood work, depending on the diagnosis. Other nontoxic-appearing, stable patients can be seen in the outpatient primary care setting for assessment of nutritional deficiencies, oncologic work-up, or autoimmune disease.
Patients with persistent symptoms affecting activities of daily living, or leading to increased falls at home, may warrant admission for physical therapy and occupational therapy evaluation. Elderly patients may be evaluated for placement in assisted living facilities or subacute rehabilitation centers better equipped to manage their physical limitations. Overall, the decision may be a coordinated conversation between the physician, patient, and family members regarding the next best step for the patient.
Summary
Uncovering the etiology of acute ataxia can save a life, halt disease progression, or improve symptom control. With earlier intervention, especially early in the disease course, most patients can regain most normal functions of daily living. It is the task of the astute physician to be aware of all the varying presentations and know what clues to look for to discover the underlying pathology. As with many of these disease processes, the presentation can be atypical, and an interprofessional team should be called to evaluate each case promptly.
REFERENCES
- Ashizawa T, Xia G. Ataxia. Continuum (Minneap Minn) 2016;22:1208-1226.
- Beristain X. Approach to the patient with gait disturbance and recurrent falls. In: Biller J, ed. Practical Neurology. 5th ed. Wolters Kluwer;2017:105-118.
- Pirker W, Katzenschlager R. Gait disorders in adults and the elderly: A clinical guide. Wien Klin Wochenschr 2017;129:81-95.
- Searls DE, Pazdera L, Korbel E, et al. Symptoms and signs of posterior circulation ischemia in the New England Medical Center posterior circulation registry. Arch Neurol 2012;69:346-351.
- Akhtar N, Kamran SI, Deleu D, et al. Ischaemic posterior circulation stroke in State of Qatar. Eur J Neurol 2009;16:1004-1009.
- Arch AE, Weisman DC, Coca S, et al. Missed ischemic stroke diagnosis in the emergency department by emergency medicine and neurology services. Stroke 2016;47:668-673.
- Venkat A, Cappelen-Smith C, Askar S, et al. Factors associated with stroke misdiagnosis in the emergency department: A retrospective case-control study. Neuroepidemiology 2018;51:123-127.
- Fasano A, Bloem BR. Gait disorders. Continuum (Minneap Minn) 2013;19:1344-1382.
- Mariotti C, Fancellu R, Di Donato S. An overview of the patient with ataxia. J Neurol 2005;252:511-518.
- Kuo SH. Ataxia. Continuum (Minneap Minn) 2019;25:1036-1054.
- Rao AK. Gait disorders. In: Louis ED, Mayer SA, Rowland LP, eds. Merritt’s Neurology. 13th ed. Wolters Kluwer;2015:107-117.
- Ataxia and disorders and cerebellar function. In: Ropper AH, Samuels MA, Klein JP, Prasad S, eds. Adams & Victor’s Principles of Neurology. 11th ed. McGraw-Hill; 2019.
- Alekseeva N, McGee J, Kelley RE, et al. Toxic-metabolic, nutritional, and medicinal-induced disorders of cerebellum. Neurol Clin 2014;32:901-911.
- Torvik A. Brain lesions in alcoholics: Neuropathological observations. Acta Med Scand Suppl 1987;717:47-54.
- Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health 2003;27:134-142.
- Deafness, dizziness, and disorders of equilibrium. In: Ropper AH, Samuels MA, Klein JP, Prasad S, eds. Adams & Victor’s Principles of Neurology. 11th ed. McGraw-Hill; 2019.
- Khan S, Chang R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013;32:437-443.
- Disorders of equilibrium. In: Simon RP, Aminoff MJ, Greenberg DA, eds. Clinical Neurology. 10th ed. McGraw-Hill; 2018.
- Ramirez-Zamora A. Approach to the ataxic patient. In: Biller J, ed. Practical Neurology. 5th ed. Wolters Kluwer; 2017.
- Javalkar V, Khan M, Davis DE. Clinical manifestations of cerebellar disease. Neurol Clin 2014;32:871-879.
- Quimby AE, Kwok ESH, Lelli D, et al. Usage of the HINTS exam and neuroimaging in the assessment of peripheral vertigo in the emergency department. J Otolaryngol Head Neck Surg 2018;47:54.
- Forbes J, Cronovich H. Romberg test. In: Stat Pearls; StatPearls Publishing: 2022.
- Zimmerman B, Hubbard JB. Clonus. In: Stat Pearls; StatPearls Publishing: 2022.
- Acharya AB, Jamil RT, Dewey JJ. Babinski reflex. In: Stat Pearls; StatPearls Publishing: 2022.
- Whitney E, Munakomi S. Hoffmann sign. In: Stat Pearls; StatPearls Publishing: 2022.
- Nonnekes J, Růžička E, Serranová T, et al. Functional gait disorders: A sign-based approach. Neurology 2020;94:1093-1099.
- Beghi E, Feigin V, Caso V, et al. COVID-19 infection and neurological complications: Present findings and future predictions. Neuroepidemiology 2020;54:364-369.
- Chan JL, Murphy KA, Sarna JR. Myoclonus and cerebellar ataxia associated with COVID-19: A case report and systematic review. J Neurol 2021;268:3517-3548.
- Inoa V, Aron AW, Staff I, et al. Lower NIH stroke scale scores are required to accurately predict a good prognosis in posterior circulation stroke. Cerebrovasc Dis 2014;37:251-255.
- Ioannides K, Tadi P, Naqvi IA. Cerebellar infarct. In: Stat Pearls; StatPearls Publishing: 2022.
- Smith T, Rider J, Cen S, Borger J. Vestibular neuronitis. In: Stat Pearls; StatPearl Publishing: 2022.
- Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504-3510.
- Touil LL, Watson GJ, Small M. Vertebral artery dissection: An unusual cause of transient ataxia, vertigo, and sensorineural hearing loss. Ear Nose Throat J 2013;92:E20-E22.
- Harati A, Oni P, Schultheiß R, Deitmer T. [Management of patients with vestibular schwannoma type IV]. Laryngorhinootologie 2020;99:613-619.
- Akdal G, Tanrıverdizade T, Şengün İ, et al. Vestibular impairment in chronic inflammatory demyelinating polyneuropathy. J Neurol 2018;265:381-387.
- Koeppen AH. The neuropathology of the adult cerebellum. Handb Clin Neurol 2018;154:129-149.
- Razavi AS, Karimi N. Longitudinally extensive transverse myelitis with unknown etiology presenting with sensory ataxia. J Case Rep Clin Images 2020;3:1032.
- Chow F. Brain and spinal epidural abscess. Continuum (Minneap Minn) 2018;24:1327-1348.
- Morgante F, Edwards MJ, Espay AJ. Psychogenic movement disorders. Continuum (Minneap Minn) 2013;19:1383-1396.
- Kumar N. Neurologic presentations of nutritional deficiencies. Neurol Clin 2010;28:07-170.
- Kumar N. Acute and subacute encephalopathies: Deficiency states (nutritional). Semin Neurol 2011;31:169-183.
- Pope S, Artuch R, Heales S, Rahman S. Cerebral folate deficiency: Analytical tests and differential diagnosis. J Inherit Metab Dis 2019;42:655-672.
- Nachbauer W, Eigentler A, Boesch S. Acquired ataxias: The clinical spectrum, diagnosis and management. J Neurol 2015;262:1385-1393.
- Cavallieri F, Fini N, Contardi S, et al. Subacute copper-deficiency myelopathy in a patient with occult celiac disease. J Spinal Cord Med 2017;40:489-491.
- van Gaalen J, Kerstens FG, Maas RPPWM, et al. Drug-induced cerebellar ataxia: A systematic review. CNS Drugs 2014;28:1139-1153.
- Hon KL, Hui WF, Leung AK. Antidotes for childhood toxidromes. Drugs Context 2021;10:1-10.
- Butterworth RF. Metal toxicity, liver disease and neurodegeneration. Neurotox Res 2010;18:100-105.
Ataxia and gait disturbances can signify a variety of conditions. The differential includes benign as well as life-threatening causes. An understanding of the pathophysiology and a thorough neurological exam are critical in making these distinctions.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
