Venous Thromboembolism (VTE) in the Hospitalized Patient
Part II: Treatment and Prevention of DVT and PE— Evolving Risk-Stratification and Prophylaxis Strategies for Emergency Medicine
Authors: Gideon Bosker, MD, FACEP, Assistant Clinical Professor, Section of Emergency Services, Yale University School of Medicine, New Haven, CT; Janet Poponick, MD, Assistant Professor, Department of Emergency Medicine, MetroHealth Medical Center, Cleveland, OH.
Peer Reviewers: Charles L. Emerman, MD, Chairman, Department of Emergency Medicine, MetroHealth Medical Center, Cleveland Clinic Foundation, Cleveland, OH; Kurt Kleinschmidt, MD, FACEP, Associate Professor, University of Texas Southwestern Medical Center, Dallas.
The treatment and prevention landscape for deep venous thrombosis (DVT) and pulmonary embolism (PE) is undergoing an evidence-driven shift of significant proportions. As the results of new, landmark clinical investigations and updated consensus statements related to venous thromboembolism (VTE) are published in the medical literature, clinical issues surrounding thrombosis care are coming into sharper focus.
Approaches to acute management and prevention of VTE are evolving for all hospital-based practitioners, from critical care specialists and hospitalists to cardiologists and, most recently, emergency medicine specialists.1-6
In fact, evolving mandates and recommendations surrounding the management of venous thromboembolic disease are destined to make as enduring an impact on the practice of emergency medicine as they have on other clinical disciplines. In this regard, there is both increasing awareness and evidence that hospitalized medical patients, especially those with heart failure, respiratory disease, serious infections, malignancy, and restricted mobility are at increased risk for sustaining DVT and/or PE, in addition to other complications and sequelae associated with their underlying conditions.1-9
The emergency department (ED) frequently is the portal of entry for the broad range of patients at high risk for acquiring or presenting with VTE—among them, individuals with restricted mobility who have sustained a hip fracture, require abdominal surgery, present with a serious medical illness, have a malignancy, or who manifest frank signs and symptoms of DVT. Consequently, emergency physicians must be skilled at identifying, assessing, risk-stratifying, and managing patients who require prevention against or therapy for VTE. When it comes to DVT treatment, a number of options are available, ranging from unfractionated heparin (UFH) to low molecular weight heparins (LMWHs).
While few physicians fail to institute appropriate treatment for acutely ill patients who present with classical findings of VTE, it should be stressed that failure to screen for and identify medical patients eligible for VTE prophylaxis frequently compromises patient outcomes in the hospital setting. In this regard, clinicians should recognize that the potential complications, cost, and morbidity associated with DVT in this patient population can be significant, and may include PE, prolonged hospitalization, and in some cases, sudden death. From an epidemiological perspective, it is estimated that as many as 10 million patients hospitalized annually for medical conditions merit screening for and prophylaxis against venous thromboembolic disease. Given these trends, it is inevitable that emergency practitioners will play a leading role in screening for, risk-stratifying, and initiating thrombosis-modifying strategies in the hospital environment.
Put simply, the venous "thrombosis crisis" rapidly is becoming a clinical focal point for emergency personnel evaluating and caring for medical patients with serious illness. It should be stressed that patients with restricted mobility associated with such conditions as congestive heart failure (CHF), respiratory failure, serious pulmonary and systemic infections, and underlying malignancy are at greater risk for sustaining the morbid sequelae and complications associated with DVT. Accordingly, the threshold for instituting prevention against DVT in this patient population must be balanced against the relatively low risk of serious complications associated with anticoagulant-mediated prophylaxis. As a general rule of thumb, if the risk of thrombosis exceeds the risk of consequential hemorrhage, prophylactic anticoagulation should be strongly considered in the emergency setting, based on consultation with the admitting hospital physician.
Since introduction of LMWHs to the United States market in 1993, their indications have continued to expand. Of special significance to emergency medicine and inpatient physicians is approval of enoxaparin for outpatient treatment of DVT, as well as approval of this LMWH for prevention of DVT in risk-stratified, hospitalized medical patients. Considering the evolving awareness of thrombosis-related issues in the hospital-based setting, accompanied by the increased focus on delivering cost-effective health care, hospital practitioners, including emergency physicians, must become informed about opportunities offered by LMWHs to decrease patient and drug monitoring costs, reduce length of hospitalization in patients with DVT, and prevent life-threatening venous thrombotic disorders.
With these considerations in mind, the following evidence-based review outlines the rationale and indications for DVT prevention and treatment, emphasizing cost-effective strategies for outpatient therapy, risk stratification of hospitalized patients at risk for VTE, and clinical care pathways that can be implemented at the institutional level for optimizing management of VTE.
A number of therapeutic strategies for VTE prevention and treatment will be highlighted, in particular, the evidence-based, risk management upgrade from UFH to LMWHs, such as enoxaparin, as the agent of choice for a wide spectrum of thrombotic disorders. Outpatient strategies for DVT treatment also will be outlined and their cost-effectiveness as compared to in-hospital management will be discussed.
The evolving, sometimes controversial role of fibrinolytic therapy for venous thrombotic conditions also will be examined. Although the effectiveness of fibrinolytic therapy in acute ST-elevation myocardial infarction and stroke has been confirmed, use of these agents is not as widespread in VTE. Nevertheless, the two principal indications for their use include massive PE with hemodynamic compromise and massive ileofemoral DVT. Finally, prophylaxis of medical patients at risk for sustaining VTE will be discussed in detail, and such studies as the MEDENOX, PRIME, and PRINCE trials, which have validated the need for enoxaparin-mediated prophylaxis in hospitalized medical patients, will be analyzed for their implications on emergency practice. The third and final part of this series will cover VTE risk stratification, assessment, and prevention from the emergency perspective.—The Editor
Overview of Anticoagulation Therapy
Despite recognition of risk factors and availability of pharmacologically effective options for prophylaxis, DVT and PE remain common causes of morbidity and mortality. It is estimated that approximately 600,000 patients per year are hospitalized for DVT in North America.10 In the United States, symptomatic PE is estimated to occur in more than 600,000 patients and may cause or contribute directly to death in up to 200,000 patients annually.11 Notably, PE is the most common cause of death in patients following total hip replacement, and has been cited as the most common cause of preventable hospital mortality. The postphlebitic syndrome, characterized by persistent leg pain, swelling, and ulcerations, affects 15 million Americans, predisposes to recurrent VTE, and may be prevented if thrombosis prophylaxis is routinely and appropriately used and DVT treated effectively.5 A cohort analysis indicates that the mortality associated with VTE may be even higher than previously estimated.12
The Role of Heparins for DVT Prevention and Management—An Evolving Pharmacological Landscape
Until recently, and since the introduction of warfarin in the 1950s, the management of VTE in the United States had undergone relatively few changes. Typically, patients with proximal, symptomatic distal DVT were hospitalized and placed at bed rest. They were given a bolus of intravenous (IV) UFH followed by a continuous IV infusion of UFH for 5-6 days until therapeutic anticoagulation with warfarin was achieved; this endpoint usually was defined as an International Normalized Ratio (INR) of 2.0-3.0. The heparin dose was adjusted to achieve an activated partial thromboplastin time (aPTT) ratio of 1.5-2.5, which correlates with a therapeutic heparin level of 0.2-0.4 IU/mL by protamine titration or an anti-Xa level of 0.3-0.6 IU/mL by a chromogenic assay.13 The strategy of dosing UFH by body weight was introduced 1993 and permitted more rapid attainment of a therapeutic aPTT.14
Although the complication rate during the hospitalization period for most patients with DVT is low, the opportunity to treat DVT on an outpatient or short-stay basis been explored in a number of clinical trials.15-18 The need to administer UFH by constant IV infusion guided by frequent aPTT measurements precluded outpatient therapy or early discharge. The opportunity to initiate outpatient or short-stay programs for DVT treatment has been made possible by the introduction of LMWHs, such as enoxaparin, which have significant pharmacokinetic advantages over UFH as result of differences in size, structure, and mechanism of action.
Structure of Heparins. LMWH and UFH are glycosaminoglycans comprised of chains of alternating residues of D-glucosamine and an uronic acid, which may be either glucoronic or iduronic acid.19 Commercially, heparins most often are prepared from porcine intestinal mucosa. UFHs are treated by chemical or enzymatic depolymerization to form LMWHs. The mean molecular weight distribution of LMWH is 4000-6000 daltons, compared to about 12,000 daltons for UFH.20 The majority of UFH molecules are greater than 18 saccharide units long; however, this is true in fewer than half of LMWH molecules. Differences in size and structure account for the distinct pharmacological actions of LMWHs.
Mechanism of Action. Heparins exhibit most of their antithrombotic effects by inactivating two important factors in the coagulation cascade: factor Xa and factor IIa (thrombin). Heparin acts as a template to which both antithrombin III and coagulation enzymes bind. One of the principal differences between UFH and LMWH is that LMWH demonstrates greater activity against factor Xa than factor IIa (4:1 to 2:1), whereas UFH has similar effects against both.
Approximately one-third of UFH and LMWH molecules have a unique pentasaccharide, enabling them to bind to antithrombin III and subsequently inhibit factors IIa and Xa. However, only those heparin molecules with at least 18 saccharide units are capable of forming a ternary complex with antithrombin III and factor IIa.19 Since only about a third of LMWH molecules are large enough to form this ternary structure, they have less effect on factor IIa but retain their antifactor Xa activity.21 In addition, both UFH and LMWH stimulate the release of tissue factor pathway inhibitor (TFPI) from the endothelium. TFPI complexes bind with and inactivate factor Xa and factor VIIa and work independently of pentasaccharide binding. Finally, heparin inactivates factor IIa by heparin cofactor II.
Pharmacokinetics. The primary pharmacokinetic advantage of LMWHs is their increased bioavailability, which results from their decreased affinity for circulating plasma proteins. UFH binds readily to histidine-rich glycoprotein, polymeric vitronectin, platelet factor IV, multimers of von Willebrand factor, fibronectin, macrophages, and endothelial cells.22 In addition, UFH may exhibit unpredictable anticoagulant activity due to the wide inter-patient variability in plasma concentrations of these heparin-binding proteins. Some of these proteins are acute phase reactants while others are released during the clotting process. To compensate for binding affinity to a broad range of circulating plasma proteins, higher doses of UFH usually are required to achieve anticoagulant activity. Moreover, frequent laboratory monitoring is required for UFHs to ensure therapeutic activity has been attained while minimizing the risks of excessive anticoagulation.
In contrast, lab monitoring usually is not required for LMWHs due to their superior bioavailability, more predictable dose response effect, and minimal effect on factor IIa. Exceptions to this caveat include patients with renal insufficiency (since both UFHs and LMWHs are eliminated renally), patients who are markedly obese or underweight, and other patient subgroups. Because LMWHs do not prolong the aPTT, monitoring of the anticoagulant response in these patients is achieved primarily by following anti-Xa levels. Such monitoring may be required in the aforementioned subgroups, which may demonstrate an unpredictable dose response effect.
Variation in plasma protein concentrations results in unpredictable renal and hepatic clearance. Because LMWHs bind less avidly to macrophages and to vascular endothelium, the renal and hepatic clearance is slower and results in a longer plasma half-life. The improved bioavailability and longer half-life of LMWHs permits these agents to be dosed on a once- or twice-daily basis.
Indications for LMWHs. LMWHs have been used in Europe and Canada for more than 10 years, during which time a great deal of clinical experience has been gained in a variety of venous and arterial thrombotic disorders. Enoxaparin, in particular, was introduced in the United States in 1993, and currently is the only LMWH approved for prevention of DVT in the medically ill patient. LMWHs are widely used for prophylaxis of DVT in patients undergoing surgery, particularly high-risk abdominal surgery patients, individuals undergoing cancer surgery, and in those patients requiring total hip and knee replacement.
The value of extended DVT prophylaxis with enoxaparin is supported by the results of a prospective, randomized, double-blind study demonstrating a 63% risk reduction in DVT at 21 days compared to placebo post-discharge after total hip replacement.23 There was no significant difference in incidence of adverse events, including major hemorrhage. Enoxaparin is the only LMWH approved by the FDA for extended prophylaxis after hospital discharge in patients with total hip replacement. Even though, when measured in drug acquisition costs, LMWHs are more expensive than UFHs, they appear to be more cost effective for DVT prophylaxis after major orthopedic surgery.24
The Sixth American College of Chest Physicians’ Consensus Conference on Antithrombotic Therapy has published recommendations for DVT prophylaxis and has identified LMWH as an acceptable pharmacological agent for most at-risk patients.25 In addition to VTE surgical prophylaxis and treatment, two LMWHs (enoxaparin and dalteparin) also are approved by the FDA for the prevention of thrombotic complications in the setting of acute non ST-elevation myocardial infarction and unstable angina.
Treatment of VTE
Cost- and outcome-effectiveness imperatives within healthcare systems require emergency physicians to be aware of the significant cost savings that, in appropriately selected patients, can accrue from outpatient management of DVT. Furthermore, given the substantial administration and monitoring costs associated with administration of IV UFH—as well as a number of studies2 suggesting a greater risk of major hemorrhage and heparin-induced thrombocytopenia (HIT) with UFH therapy—clinicians and hospital pharmacists are re-evaluating their initial choices for anticoagulant therapy and prophylaxis, with a growing trend toward adoption of LMWH as the anticoagulant of choice for management of VTE.
More than 20 published studies have evaluated the safety and efficacy of various LMWHs for treatment of VTE and, as a result, these agents are playing an increasingly pivotal role in treatment of these acute—and sometimes life-threatening—conditions. One well-designed meta-analysis of the literature concluded that LMWHs were at least as effective and safe as UFH for DVT management.26 In a prospective study of 500 patients, one group compared outpatient enoxaparin administered 1 mg/kg subcutaneously (SC) bid vs. UFH administered in the hospital by IV infusion with aPTT monitoring for the treatment of DVT.15 All patients received warfarin. Patients randomized to enoxaparin spent an average of only 1.1 days in the hospital compared to 6.5 days for UFH. One hundred twenty patients receiving enoxaparin were managed entirely out-of-hospital, and there were no significant differences in major bleeding between the two groups.
Another research group randomized 400 patients to nadroparin (an LMWH not available in the United States) vs. UFH.16 Rates of recurrent VTE between the two groups were not significantly different; about 40% of the patients assigned to nadroparin did not require hospitalization. Moreover, bleeding complications were less frequent in the LMWH-treated patients. Another European multicenter trial demonstrated that 253 patients randomized to dalteparin administered once daily vs. UFH did not manifest a statistical difference in progression of their DVT distal to the inguinal ligament.17 Another investigation compared the LMWH logiparin (not available in the United States) to IV UFH and demonstrated equal effectiveness and safety in the treatment of proximal DVT.18 Finally, in a review of the literature, one analyst concluded that, overall, LMWHs demonstrate clinical superiority to UFH in the treatment of DVT, when aggregating a constellation of potential benefits, including efficacy, safety, convenience, and total resource costs.27
Additional support for LMWHs in DVT management was forthcoming in a well-designed, prospective study of 900 patients with DVT with or without PE, in whom enoxaparin was found to be as safe and effective as UFH.28 These results, in combination with the aforementioned outpatient vs. inpatient study comparing enoxaparin and UFH, were sufficiently compelling to induce the FDA to approve enoxaparin for: a) inpatient treatment of DVT with or without PE and; b) outpatient treatment of appropriately selected patients with DVT.
Outpatient or Short-Stay Treatment of VTE. Opportunities and indications for managing low-risk patients with DVT in the outpatient setting should be considered for patients presenting to the ED. Although evaluation of patients suspected of having VTE has been described previously, a number of points should be stressed. No patient should be subjected to the risk, expense, and/or inconvenience of anticoagulation therapy without clinical confirmation of VTE. When performed by a technically proficient examiner, duplex ultrasonography is considered sufficient for establishing the diagnosis of DVT. However, if the results of duplex ultrasonography are indecisive, venography remains the unambiguous standard for confirming the diagnosis.
In the case of PE, diagnostic tests include ventilation/perfusion scan, pulmonary angiography, spiral computerized tomography, and gadolinium magnetic resonance. D-dimer (a fibrin split product) testing is considered a sensitive but not specific test for the diagnosis of VTE. Therefore, normal plasma levels (< 500 mcg/L by enzyme-linked immunosorbent assay) provide excellent, negative predictive value in patients suspected of having PE, especially in those patients with a low clinical suspicion for the disease. A systematic approach to diagnosis of PE was presented in Part I of this series.
As a rule, baseline laboratory evaluation for VTE should include a complete blood count (CBC) including platelet count, aPTT, and PT/INR. The physician should evaluate the patient’s candidacy for thrombolytic therapy, especially in younger patients with acute symptomatic iliofemoral thrombosis. Patients should be considered for a hypercoagulable state (thrombophilia) work-up (e.g., protein C, protein S, anti-thrombin III deficiency, factor V Leiden, antiphospholipid antibodies) if there are no obvious causes for their DVT, if they are young, or if associated clinical findings suggest current or past hypercoagulability states.
Those who might benefit from a hypercoagulability work-up include patients with family history of VTE, development of DVT in the upper extremities, or DVT occurring without obvious provoking factors such as immobility, trauma, or surgery. Concurrent treatment with heparin and/or warfarin may complicate work-up of these patients because anticoagulants can interfere with test results. For example, warfarin decreases protein C and S levels. Similarly, an acute VTE with comorbid conditions may falsely alter the levels due to the increase in acute phase reactants that accompany stress. Because the cost of testing for hypercoagulable states can be substantial, and because the results normally do not affect the acute treatment decision, it is suggested that the hypercoagulable state work-up be performed about 2-3 weeks after the cessation of therapy. Consultation with the laboratory director or hematologist may be helpful in determining the scope and timing of any anticipated work-up.
Because cancer is a common cause of a hypercoagulable state, patients presenting with unprovoked VTE should be considered for possible evaluation of occult malignancy. At a minimum, these patients should have a thorough history and physical examination and subsequent clinically appropriate cancer screening, such as mammography and fecal occult blood testing. Estrogen replacement therapy or oral contraceptives must be discontinued, as they are absolutely contraindicated during the course of therapy for VTE.
As a rule, only those patients with uncomplicated or low-risk DVT should be considered eligible for outpatient treatment. The diagnosis of DVT should be confirmed and a determination should be made that the patient is a candidate for LMWH treatment. There are some institutions that initiate LMWH in patients with symptoms of DVT who present late at night and delay the ultrasound until morning. Although several LMWHs have been evaluated for outpatient treatment of DVT, currently only enoxaparin has gained FDA approval for this treatment indication. For in-hospital treatment of DVT with or without PE, the approved dose of enoxaparin is either 1 mg/kg SC q 12 hours or 1.5 mg/kg SC q day. For outpatient treatment, the only FDA-approved dosage is 1 mg/kg bid. Enoxaparin is available in 30, 40, 60, 80, and 100 mg pre-filled disposable syringes. Multi-dose vials that permit more precise weight-based dosing also are available.
Hospital vs. Outpatient Therapy for DVT
Because of the cost associated with in-hospital treatment of DVT, critical pathways now emphasize outpatient management with enoxaparin when appropriate. There is considerable variation in the management of DVT, even within a single hospital, and clinical pathways may serve to decrease cost and length of stay.29
In this regard, LMWH represents a major advance in the treatment of DVT, especially with the availability of the LMWH enoxaparin, for treatment of DVT.30 Some studies that have compared standard IV UFH to subcutaneous LMWH show that LMWH has improved antithrombotic effects and has fewer adverse consequences.31-33 In this regard, one meta-analysis study demonstrated that LMWH reduced thromboembolic complications, clinically important bleeding, and mortality when compared to unfractionated heparin.34 In addition, because LMWH can be given subcutaneously once or twice a day without need for coagulation tests, home treatment with enoxaparin for DVT has become a clinical reality.
There is evidence that suggests LMWHs administered subcutaneously in fixed doses adjusted for body weight and without laboratory monitoring may be more effective and safer than adjusted-dose standard heparin. In a major meta-analysis, LMWH reduced symptomatic thromboembolic complications by 53%, clinically important bleeding by 68%, and mortality by 47% when compared to standard heparin.34 A separate meta-analysis, including more than 2000 patients and 16 controlled trials, found that LMWH significantly reduced thrombus extension (odds ratio: 0.51) and demonstrated a trend toward decreased PE, fewer major bleeds, and lowered mortality.33 Of particular concern to the internist, heparin-induced thrombocytopenia (HIT) is less common with LMWH than with UFH.35
The only LMWH currently approved for both outpatient and inpatient treatment of DVT in the United States is enoxaparin. The approved dose of enoxaparin for inpatient treatment of DVT, with or without PE, is 1 mg/kg q 12 hours SC or 1.5 mg/kg SC qd. The dose of enoxaparin for outpatient therapy of DVT without PE is 1 mg/kg q 12 hours SC.36,37
The safety and use of home treatment of DVT has undergone rigorous investigation. In two recent studies, authors compared adjusted-dose IV standard heparin administered in the hospital to fixed-dose subcutaneous LMWH administered twice daily at home.37,38 In both studies, home treatment with LMWH compared favorably with standard in-hospital therapy. Patients treated at home—or with a short hospitalization with early discharge to home—spent 67% less time in the hospital and had greater physical activity and social functioning than their standard heparin cohorts.38 Some patients had professional home health care assistance with their injections; all had careful follow-up.
Outcomes and Implementation Strategies. The potential pharmacoeconomic advantages of out-of-hospital treatment with enoxaparin include fewer admissions, increased patient comfort, and decreased overall costs. A disadvantage is that patients have to be evaluated carefully to identify those who would be more safely treated in the hospital. In addition, much of the responsibility for treatment would be shifted from medical personnel to the patient and family, requiring self-administration of anticoagulants, self-monitoring for safety and efficacy, and compliance with clinic appointments for dosage adjustments of oral anticoagulants.
Initial treatment of DVT at home should follow a protocol in which all aspects of the treatment are defined clearly for the family and follow-up physician. Ideally, each ED should develop a treatment protocol that is written in advance by a team of medical professionals experienced in the treatment of DVT. The protocol should include criteria for patient selection, enoxaparin and warfarin therapy, patient and caregiver education, and monitoring. Each medical facility will need to develop a protocol that fits its own practice patterns.
One of the most important goals is developing criteria to identify patients who qualify for home-based treatment of DVT. As mentioned, most studies evaluating LMWH for outpatient therapy have excluded patients with a high risk of bleeding (malignant hypertension, peptic ulcer disease, recent surgery, known bleeding disorders, thrombocytopenia, high risk of falling), a high risk of recurrent thrombosis (previous DVT, pregnancy), and suspected PE.36,39 In addition, patients who resided a long distance from follow-up medical care, who were unable to care for themselves or did not have a competent caregiver in residence, and who were simply too ill to stay at home were not considered candidates for home-based treatment of DVT. Patients had to be willing to participate in their care, including self-administration of medications and follow-up for warfarin dosage adjustment when necessary. With these strict guidelines for patient selection, about 22-58% of patients screened in various studies were considered eligible for home-based treatment of DVT with enoxaparin.36,39
Patient Instructions. Detailed instructions must be provided by the emergency medicine team. The patient or caregiver must be taught to administer the medication, monitor for adverse reactions and efficacy, and perform any other self-care deemed necessary (such as bed rest, leg elevation, and use of compression stockings). The patient or caregiver also must know what steps to take in the event of a complication. Instruction should begin immediately after diagnosis and can be provided by a nurse, a pharmacist, or both. Written instructions also should be provided.
Monitoring home-based treatment of DVT with enoxaparin should include compliance, subcutaneous injection technique, local adverse effects from the injections, signs of bleeding, signs of recurrent thrombosis, and initiation and monitoring of warfarin therapy. Much of this monitoring can be done by the visiting nurse, who should see the patient daily during the initial treatment period of 5-9 days.
It also may be useful for a nurse, a pharmacist, or a physician from the treatment team to telephone the patient or caregiver periodically to ensure that treatment is going as planned and that there are no complications. For patients selected to undergo home-based treatment with enoxaparin, the LMWH should be administered for at least five days, and warfarin can be started on the same day as the LMWH or the day after. Blood should be drawn daily to monitor the prothrombin time for the first few days; the INR should be between 2.0 and 3.0 for two consecutive days before the LMWH is stopped.
Pharmacoeconomic Considerations of Outpatient Therapy. With approval for DVT treatment, enoxaparin represents a breakthrough in out-of-hospital management of venous thromboembolic disease. It should be stressed that this approach is part of a larger movement to reduce total outcome costs. In a health care environment that puts a premium on effective therapy implemented in a cost-optimizing manner, outpatient DVT treatment with enoxaparin has special utility for emergency physicians who, in many cases, will be called upon to risk-stratify patients as to their suitability for outpatient treatment of DVT.
The safety and efficacy of taking LMWH at home has been confirmed in clinical trials in which patients with acute proximal DVT were randomized to receive either IV standard heparin in the hospital or subcutaneous LMWH administered primarily at home.29-33 One economic evaluation was conducted by prospectively collecting data on resource use and health-related quality of life (Medical Outcomes Study Short-Form 36) on the 300 patients who formed the trial stratum presenting with proximal vein thrombosis as outpatients, of whom 151 received standard heparin and 149 received LMWH. The primary viewpoint of the analysis was societal, and costs included health care costs, patient travel costs, and productivity costs as a result of time off work. Costs were assessed over a period of three months from randomization. Quality of life was assessed as the change in Short-Form 36 domain scores from baseline to day 7 for each treatment group (all costs are in 1997 Canadian dollars).
There were 11 recurrent thromboembolic events and 1 bleed in the 151 patients who received standard heparin; the corresponding data for the 149 patients receiving LMWH were 10 and 4, respectively. The mean cost per patient who received standard heparin was $5323 (Canadian dollars) as compared with $2278 for LMWH, a total societal cost savings per patient using LMWH of $3045 (95% confidence interval, $2012-$4050). There was no difference in quality of life between the two groups except for the domain of social functioning, where a greater improvement from baseline to day 7 was observed for the LMWH group vs the standard heparin group (P=.005). For patients with acute proximal DVT, treatment at home with LMWH is less costly than hospital-based treatment with standard heparin.
Warfarin. In documented DVT, warfarin therapy is initiated by the patient’s primary physician upon admission to hospital, or alternatively, by the emergency physician in consultation with the primary physician in those cases where outpatient therapy is deemed appropriate. Warfarin may be given 2-4 hours after the initial subcutaneous dose of enoxaparin. Warfarin temporarily can cause a hypercoagulable state because it produces depletion of protein C and protein S, both of which have a short half-life.
A commonly used approach is to begin with a warfarin dose estimated to be the patient’s eventual maintenance dose. For most patients, this will be 5 mg daily with subsequent doses to be adjusted based on the results of the INR to achieve a goal INR of 2.0-3.0. Higher loading doses have not produced more rapid attainment of targeted INR and run the risk of increased bleeding. Although not universally agreed upon, patients known to have a hypercoagulable state caused by the antiphospholipid antibody syndrome may require an INR of 3.0-3.5.40-42
The multiple drug-drug and drug-food interactions associated with warfarin warrant an attempt to keep the patient’s vitamin K intake and concomitant drug usage stable. Warfarin should be administered in the afternoon or evening to allow for morning INR results to return so that dosing can be adjusted appropriately. There is no good supporting evidence for the value of routine bed rest, and bed rest with leg elevation would be appropriate for those patients with significant leg pain and swelling until such symptoms resolve.40
Patient Education. Patient education forms the cornerstone of outpatient anticoagulation management with LMWH. The patient needs to be instructed on signs and symptoms of PE, bleeding, or extension of DVT. Also, the patient needs instruction on proper technique of LMWH administration as well as drug-drug and drug-food interactions with warfarin. Case managers are helpful in securing third-party reimbursement and arranging for home health agency (HHA) visits if required. Many HHAs are equipped today with portable finger-stick monitoring devices so that the PT/INR can be obtained immediately and the results called in to the pharmacist or physician for warfarin dosing changes.
Platelet counts do not need to be performed routinely if the total course of LMWH administration is expected to be fewer than seven days. Once the INR is greater than 2.0 for two consecutive days, LMWH can be stopped and the patient continued on warfarin. Although there is some debate regarding the length of warfarin therapy, it generally is recommended that the minimum duration is three months—although recurrence rates are less when treatment is extended to at least six months for patients with idiopathic VTE.32
Patients who develop worsening signs and symptoms of DVT extension such as increasing swelling and pain, and those patients who show evidence of PE should be considered for hospital admission. The mortality rate of patients with DVT who are anticoagulated promptly and effectively is extremely low. A meta-analysis indicates the rate of fatal PE during 5-10 days of heparin and three months of oral anticoagulants is around 0.4%.33
Anticoagulation Therapy for VTE
Until recent confirmation that LMWH produces comparable—and in many cases, more cost-effective outcomes in VTE than UFH—initial treatment of patients with acute DVT consisted of IV UFH, followed by warfarin therapy for at least 3-6 months.43-45 However, as growing experience and data have emerged with LMWHs, a greater percentage of patients now are being treated with enoxaparin in the hospital and/or managed as outpatients, frequently with short hospital stays. In general, treatment regimens for DVT and PE are the same, as both are manifestations of the same disease process, VTE. The goals of anticoagulant therapy include prevention of PE, recurrence of DVT, and propagation of thrombus.
Unfractionated Heparin. When the decision is made to treat patients with DVT or PE with UFH, the anticoagulant should be started as soon as possible after exclusion criteria for anticoagulant therapy have been considered.46,47 Weight-adjusted protocols represent the current standard dosing UFH. In most patients, the weight-adjusted dosage regimen is effective at achieving a partial prothrombin time (aPTT) of 60-80 seconds, or 1.5-2.5 times the control. The usual dose is an initial bolus of 80 U/kg followed by a continuous infusion at 18 U/kg/hour. Further adjustments are based on the aPTT after 6 hours of therapy (See Table 1).47 UFH is continued for 5-7 days.
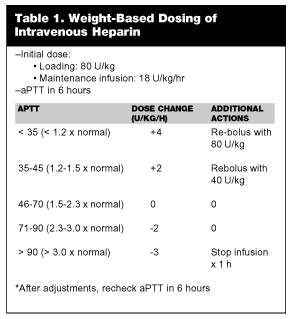 |
Oral anticoagulation with warfarin generally is initiated on the first or second day of therapy. When dose adjustment of warfarin achieves an appropriate level of anticoagulation, the heparin may be stopped. This approach is effective, reducing hospitalization by 3-5 days, and the recurrence rate of VTE is low.13,48 Some investigators have found that inadequate anticoagulation with UFH correlates with a rate of recurrence.49 In fact, the rate of DVT recurrence may be 15 times higher in those patients who fail to achieve adequate anticoagulation in the first 24 hours.50 Therefore, achieving adequate anticoagulation within the first day of therapy is important if UFH therapy is selected, and the weight-adjusted nomogram represents the best strategy for achieving this goal.
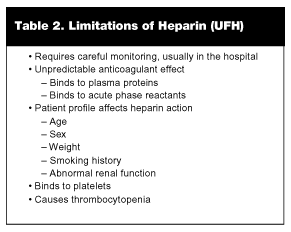
Despite a long and established tradition using UFH to treat VTE, it should be stressed that there are many limitations to UFH therapy (See Table 2). First, UFH requires careful laboratory monitoring of the aPTT to ensure adequate anticoagulation is achieved, with some studies showing that up to 62% of patients fail to achieve clinically appropriate levels of anticoagulation within the first 24 hours. Moreover, UFH therapy is unpredictable in some patients due to binding to plasma proteins. In addition, the anticoagulant effect of UFH may vary among patients depending on age, sex, weight, smoking history, and renal function. Finally, UFH can increase the risk of bleeding by reducing platelet counts. Heparin-induced thrombocytopenia (HIT) is an antibody-mediated adverse reaction that occurs in a small percentage of patients. It may be associated with arterial thrombosis and extension of thrombus.13,50,51
 |
Relative contraindications to UFH include active bleeding or the potential to bleed acutely, such as active peptic ulcer disease, recent surgery, trauma, intracranial hemorrhage, or prior history of heparin-induced thrombocytopenia. If bleeding occurs during therapy, protamine sulfate will reverse the effects of UFH.
Bleeding. Hemorrhage is the most frequent and significant side effect of heparin. Animal studies have shown hemorrhage is more frequent with UFH than LMWH when given in equipotent doses.20 LMWHs have less inhibition of platelet function and less interaction between platelets and endothelial walls than UFH. Animal studies have shown reduced bleeding in those LMWHs with higher anti-Xa:anti-IIa ratios. Patients at risk of bleeding include those with peptic ulcer disease and those who have had recent surgery. All patients should be screened at the time of VTE diagnosis, and patients at excessive risk for bleeding should be excluded from consideration for outpatient management.
Patients should be educated to promptly report any symptoms of bleeding, such as tarry stools, coffee-ground emesis, weakness, pallor, and fatigue. If bleeding is minor, holding LMWH usually is adequate. If bleeding is major, the effects of LMWHs can be at least partially reversed with protamine sulfate, a basic protein that neutralizes heparin’s anti-IIa activity. It should be administered milligram for milligram and given slowly over 10 minutes as it may cause hypotension. The total dose should not exceed 50 mg. One study comparing the effects of protamine on UFH and LMWH showed near complete reversal of anti-Xa activity and aPTT.52 However, in patients with prolonged aPTT on LMWH, protamine had a minimal effect on anti-Xa.
Low Molecular Weight Heparin. Because it produces comparable outcomes with a lower risk of major hemorrhage, reduced risk of thrombocytopenia, and lower utilization of both human and laboratory resources, LMWHs are replacing UFH as the treatment of choice for DVT and PE. Numerous studies confirm that LMWH is safe, effective, and cost-prudent in appropriately selected patients. As emphasized earlier, LMWHs are prepared by chemical or enzymatic depolymerization of UFH and exerts their anticoagulant effects by inactivation of Factor Xa. In addition, LMWH has a small effect on thrombin and a negligible effect on the aPTT; therefore, monitoring usually is not necessary, except is special patient subsets (i.e., the morbidly obese, patients with severe renal failure, and pregnant patients).50,51,53
The smaller size of the molecules (molecular weights ranging from 4000-5000 daltons) reduces significantly binding to plasma proteins, acute phase reactants, and vascular endothelium. The result is a more predictable anticoagulant effect and a longer half-life. In addition, LMWH produces minimal effects on platelets, in turn decreasing the incidence of thrombocytopenia.50,53 Multiple studies document safety of LMWHs with regard to bleeding complications, and demonstrate comparable efficacy with regard to recurrence rate when comparing UFH with LMWHs for treatment of VTE.13,49,50,53
At present, tinzaparin (Innohep®) and enoxaparin (Lovenox®) are the two LMWHs approved in the United States for treatment of DVT. Enoxaparin is used more widely, is better studied, and carries more indications across the spectrum of thrombosis-related disorders, including prophylaxis of DVT in medically ill patients. (See below.) The approved dose of enoxaparin for inpatient treatment of DVT, with or without PE is 1 mg/kg q 12 hours SC or 1.5 mg/kg SC qd. The dose of enoxaparin for outpatient therapy of DVT without PE is 1 mg/kg q 12 hours SC; the dose for prevention of DVT in medical patients is 40 mg SC once daily.36,37 The dose for tinzaparin is 175 anti-Xa IU/kg of body weight SC once daily for at least 6 days.
Administration of LMWHs is by the subcutaneous route at a fixed dose based on weight; the anticoagulant is given either once or twice daily. (See Table 1.) Because LMWHs are excreted by the kidneys, these agents are more difficult to use in patients with severe renal insufficiency. Monitoring generally is neither required nor performed, but may be required in those with renal failure (creatinine clearance less than 30 mLs/minute) or morbid obesity. Unfortunately, many hospital laboratories do not measure anti-Factor Xa levels that may be required for monitoring selected patients on LMWH.13,48,51
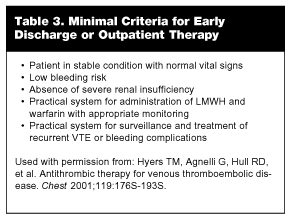
Although the drug acquisition costs of LMWH itself are greater than those for UFH, the overall resource costs (laboratory monitoring, nursing drug administration time, pharmacy preparation time, opportunity for outpatient therapy, etc.) generally are lower with LMWHs than total costs associated UFH. It is estimated that about 5-6 days of hospital cost, on average, can be saved with outpatient therapy.13,48,50,53 Initial studies evaluating the suitability of patients for outpatient treatment with DVT were skewed toward conservative inclusion and exclusion criteria. As safety and efficacy data mounted, the eligibility criteria for outpatient management have been relaxed significantly.13 (See Table 3.)
 |
Ensuring that logistical support is available is an important criterion for initiating home-based therapy with enoxaparin. The patient or family must be capable of administering the subcutaneous injections or have access to home health nursing care. Renal insufficiency usually is defined as a creatine greater than 3 mg/dL and is one exclusion criterion for home-based treatment.22,54 Bleeding risk is characterized by surgery within four weeks, prior hemorrhagic stroke, ischemic stroke within six months, or intracranial malignancy.22,54 Those who are actively bleeding or have active peptic ulcer disease are best managed in the hospital. Initial studies of evaluating safety and efficacy of LMWH were performed almost exclusively on patients with DVT only and excluded patients with symptomatic PE. The presence of symptomatic PE is an indication for in-hospital management.55
The benefit of LMWH in DVT was demonstrated in a recent randomized study documenting regression of thrombus in patients receiving reviparin. Thrombus regression was documented by venograms performed initially and after 21 days of therapy. Mortality and bleeding complications were similar in patients receiving per reviparin twice daily and UFH. In addition, thrombus regression was significant when reviparin twice-daily was compared to UFH (53.4% vs 40.2%, respectively).56
The largest study currently available randomized more than 1000 patients to receive LMWH or UFH, followed by a coumarin derivative for 12 weeks.57 Approximately one-third of patients had PE. The outcome variables studied were recurrent events, bleeding, or death. The results of the two groups were very similar with respect to outcome variables. The investigators concluded that LMWH and UFH are equally safe and effective for treatment of VTE.
The most recent meta-analysis comparing LMWHs with UFH evaluated 13 studies conducted throughout the 1990s.58 Each study was a randomized controlled trial of patients diagnosed with VTE. A number of LMWHs were used by different investigators. Notably, there was no significant difference in outcomes between the two therapies for recurrent VTE, major or minor bleeding, or thrombocytopenia. However, there was a statistical difference for risk of mortality favoring LMWH. Once-daily regimens were as safe and effective as twice daily regimens. However, there was no direct comparison among products or regimens. Treatment setting did not influence outcome or recurrence; however, inpatient therapy may be associated with a lower risk of major bleeding.
Based on the results of published clinical trials, reduction in overall resource costs, and depth of clinical experience, the LMWH enoxaparin should considered the initial option of choice for treating VTE; this recommendation applies whether the patient is being treated as an inpatient or outpatient. Clearly, one important advantage of enoxaparin is that appropriately selected patients can be treated as outpatients, reducing hospital costs significantly.22,59 It should be emphasized that individuals managed as outpatients require close monitoring and appropriate support from family and home health care providers. At present, the role of LMWHs for treatment of massive PE has not been thoroughly studied. Moreover, direct comparisons among LMWH products are lacking, and this issue requires further investigation.
Warfarin. Oral anticoagulation is generally safe and effective for preventing thrombus formation. Warfarin is the most commonly used coumarin preparation. It is well-absorbed from the gastrointestinal tract and transported to the liver. Warfarin inhibits the synthesis of vitamin K-dependent factors II, VII, IX, X and protein C and S. Warfarin requires several days to achieve efficacy.13
Accordingly, warfarin usually is started on day 1 or 2, along with LMWH or heparin therapy. The initial dose is 5 mg per day and adequacy of anticoagulation is monitored using the INR, with the goal being a therapeutic range INR between 2.0-3.0.13 Beginning oral therapy early in the course of LMWH or heparin decreases the length of UFH or LMWH therapy to an average of about 7 days. Heparin or LMWH may be discontinued once warfarin achieves a therapeutic INR. Warfarin never is used alone in the treatment of VTE as it may cause a paradoxical increase in hypercoagulability and increase the risk of recurrent VTE.
Multiple drugs and foods can interact with coumarin therapy and alter the state of anticoagulation. Instructions on maintaining a stable diet with a consistent amount of vitamin K is important, as well as instructions to avoid alcohol.13,47 Warfarin is considered teratogenic and, therefore, should not used in pregnancy. Although warfarin generally is well-tolerated, in individuals with partial protein C deficiency, microvascular thrombosis leading to skin necrosis may occur, requiring discontinuation of therapy. Vitamin K is the antidote for over anticoagulation produced by a coumarin preparation. For serious bleeding, fresh frozen plasma or cryprecipitate should be administered to reverse the effects of warfarin.
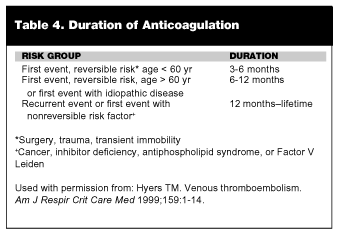
Generally, warfarin is continued for at least 3-6 months after VTE. The optimal duration of anticoagulation has not been studied in a well-designed randomized trial. However, the general recommendations are listed in Table 4.47 Although the ED physician is not following such patients, many patients present to the ED with a history of VTE and may be taking warfarin. It is important to have a knowledge regarding duration of therapy to ensure compliance, evaluate complications, or assessing new symptoms.
 |
Anticoagulation: Complications and Special Considerations
Heparin-Induced Thrombocytopenia (HIT). There are two forms of thrombocytopenia associated with heparin use. The early form is benign and reverses despite continued heparin use. The severe form is called HIT. This variant typically does not occur until day 5 of heparin therapy and is an autoimmune reaction. The body forms heparin platelet factor 4-dependent IgG antibodies that bind with platelet antigens.19 This immune complex results in thrombocytopenia and/or paradoxical thrombosis. If a patient has been exposed to heparin within the last three months, HIT can occur within 24 hours.
There is evidence that the risk of HIT is less with LMWHs than with UFHs. In one large study, the incidence of HIT at more than four days post-initiation of therapy was higher in the UFH group than the LMWH group (2.7% vs 0%). When thrombocytopenia is defined as a drop in platelets of greater than 50% in more than four days, the incidence was 5.7% and 0.9%, respectively.60,61 HIT is a clinicopathological diagnosis. Platelet activation assays using washed platelets have a sensitivity and specificity of 90% for detection of HIT antibodies. Antigen assays using enzyme-linked immunosorbent antibodies (ELISA) to detect antibodies against heparin/PF4 complexes yield sensitivities and specificities of 80% and 90%, respectively.
HIT rarely presents within five days of heparin administration, and platelet counts are not recommended routinely if the duration of heparin administration is expected to be seven days or fewer and the patient has had no heparin exposure within the previous three months. If the platelet count falls more than 50% from baseline or if the absolute platelet count falls below 100,000 /mL3, LMWH should be held pending laboratory confirmation of HIT. If the patient develops HIT and requires continuing anticoagulation, recombinant hirudin should be considered.
Lipid Effects. Heparins exert lipolytic activity and lipase enzymes, including lipoprotein lipase. In one study, total cholesterol increased by about 20% in groups given UFH or LMWH for 3-6 months.62 HDL levels increased more in the UFH group, and LDL increased more than twice that in the LMWH group although this difference was not statistically significant. Since the duration of UFH or LMWH therapy for VTE typically is a few days, these lipid effects are not clinically important.
Osteoporosis. Heparins augment PTH-stimulated bone resorption and stimulate osteoclasts. Generally, increased molecular size and the degree of sulfation are major determinants of heparin’s ability to promote bone resorption. Animal studies have shown decreased calcium loss in fetal rat calvaria with LMWH compared to UFH.63 Also, spinal fractures occur less frequently in humans on long-term LMWH compared to UFH.63 From these data, it would be expected that LMWHs would result in lower risk of heparin-induced osteoporosis.
Other Side Effects. Skin lesions associated with heparin use include erythematous papules, skin necrosis, and urticaria. Reactions such as asthma, tachycardia, tachypnea, conjunctivitis, rhinitis, angioedema, and shock are less common. Long-term administration of heparin rarely may cause hypoaldosteronism due to the inhibition of aldosterone synthesis.19
Renal Insufficiency. LMWHs, like UFHs, are eliminated through the kidneys. It is prudent in patients with renal insufficiency to adjust the dosage accordingly. Data are scarce regarding dose modifications in patients with serum creatinine of greater than 2 mg/dL. LMWHs might be best administered at reduced doses or increased intervals and monitored with anti-Xa levels. If anti-Xa levels are not readily available, reduced doses of UFH should be given and the APTT monitored accordingly.
Obese Patients. In most studies, LMWH has been dosed on the basis of actual body weight. Data on pharmacokinetics and dosing guidelines in obese patients are scarce. In patients weighing more than 130 kg, consider using a modification toward ideal body weight (IBW). A commonly used formula to adjust for ideal body weight is found below:
- IBW for men = 50 kg + (2.3 kg × [inches > 5 feet])
- IBW for women = 45 kg + (2.3 kg × [inches > 5 feet])
- If actual body weight (ABW) < IBW, enoxaparin dosing weight = ABW
- If ABW > IBW, enoxaparin dosing weight = IBW + 0.3 (ABW-IBW)
Patient Acceptance. Some patients may be hesitant to have an abbreviated hospitalization or outpatient treatment for a potentially life-threatening condition such as DVT. At least one clinical study investigated patient compliance, acceptance, and satisfaction with such a protocol. In a prospective cohort of 113 consecutive patients presenting with acute DVT, 89 were treated at home with LMWH.15-17 During the study, one patient died from a combination of PE and major bleeding. No other patient died during the three-month follow-up. One patient developed bleeding that required readmission to the hospital, and five patients developed recurrent DVT. All had active malignant disease and developed their recurrence 2-12 weeks into their course of oral anticoagulation. Of the subjects who completed the satisfaction questionnaire, 75 of 82 (91%) were pleased with home treatment; 44 of 63 (70%) felt comfortable with the self-injection of LMWH; and 71 of 77 (92%) were satisfied with the support and instructions they received during their outpatient management.15-17
Interchangeability of LMWHs. With increasing use of LMWHs, there is interest as to whether these LMWHs are truly clinically distinct or whether they can be used interchangeably. One group has demonstrated distinct pharmacologic and biochemical profiles among LMWHs.63 The Fifth American College of Chest Physicians’ Consensus Conference on Antithrombotic Therapy states that although the various LMWHs have similar profiles, they may not be interchangeable clinically.19 Statements by other organizations, including the World Health Organization and the American College of Cardiology, concur with this view. Each is produced through a distinct depolymerization process resulting in products with varying molecular weights, anti Xa:anti IIa activity ratios, release of tissue factor pathway inhibitor, bioavailability, and plasma half-lives. For these reasons, the FDA has stated that LMWHs cannot be used interchangeably.64 Due to the ever-present threat of litigation, and until more trials comparing LMWHs are available, it would seem prudent to limit the use of LMWHs to those agents that are FDA-approved for their respective indications.
The Role of Thrombolytic Agents in VTE
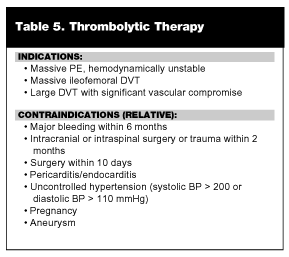
Thrombolytic agents dissolve thrombi by activating plasminogen to plasmin, which degrades fibrin to smaller peptides. Currently, there are two indications for thrombolytic therapy in VTE: 1) massive PE with associated hemodynamic compromise and; 2) massive ileofemoral DVT. (See Table 5.)13,46,47 Massive PE may present as acute dyspnea, chest pain, syncope and/or sudden death.47,65 A massive thrombus in the pulmonary vasculature can cause a precipitous increase in pulmonary artery pressure, strain on the right ventricle, and right ventricular dysfunction. Early in the course of a large PE, right ventricular dysfunction without systemic hypotension may be seen. Currently, it is controversial as to whether thrombolytics provide documented outcome advantages in this group of patients.66,67
 |
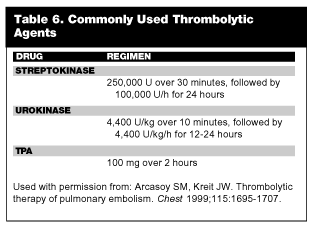
Among thrombolytic agents, streptokinase, urokinase, and tissue plasminogen activator (tPA) currently are approved for treatment of VTE. (See Table 6.) All agents appear to produce comparable results as far as degradation of thrombi.67 The two-hour tPA regimen produces more rapid lysis of clot and a more rapid return to normal hemodynamics, although perfusion scans show no difference in thrombus resolution at 36 to 48 hours after treatment.67 Recently, a two-hour infusion of streptokinase 1.5 million IU was equally effective when compared to tPA.68 Thrombolytic therapy should be initiated within 24 hours of onset of symptoms. Unlike other thrombolytic protocols used in acute coronary syndromes, heparin is not given concurrently with thrombolytic agents. The recommendation is that aPTT be checked at the conclusion of thrombolytic infusion. When the aPTT falls below 80 seconds, heparin may be started.66
 |
Thrombolytic therapy also has been shown to decrease the pain and swelling in acute DVT. However, it has not been shown to decrease the incidence of post-thrombotic syndrome. At this time, more studies are needed to evaluate outcomes for thrombolytic therapy DVT, especially since when compared to heparin alone for treatment of DVT, thrombolytics are associated with a three-fold increased risk of serious bleeding.66,69 When administered for treatment of massive PE, thrombolytics have been shown to reverse right ventricular dysfunction and lower pulmonary artery pressures by reducing overall clot burden. Unfortunately, conclusive trials are not available addressing the issue of overall survival.70
For patients with large PE resulting in right ventricular dysfunction, but who have normal systemic blood pressure, there is no unanimous consensus on optimal therapy. Unfortunately, no large trials are available to validate the use of thrombolytics in these patients,67 although one recent retrospective study attempted to draw conclusions regarding this question.71 Heparin therapy was compared to thrombolytic therapy in hemodynamically stable patients with right ventricular dysfunction. While the thrombolytic group had a greater improvement in perfusion scans, bleeding complications were more frequent, including intracranial bleeding. No difference was found in the recurrence rate of PE.71
Although controversy exists and experts differ in opinion, in general thrombolytics are indicated for massive PE with hemodynamic instability or massive DVT. Contraindications to thrombolytic therapy are the same as those applied to patients with stroke or myocardial infarction. (See Table 5.) It should be emphasized that there is a significant risk of bleeding with the use of thrombolytic agents; overall, there is a 1-2% risk of intracranial bleeding. Until outcome studies are performed in randomized control trials, thrombolytics should limited to patients with unambiguous indications for their use as previously described.
Caval Filters
Inferior vena caval (IVC) filters are indicated in patients with contraindications to anticoagulation and in those individuals who develop complications while receiving anticoagulation.72-75 IVC filters also may be useful for those at high risk of mortality from recurrent PE. Various filters are available on the market with the most well-known being the Greenfield filter. The IVC filter is inserted through the internal jugular vein or femoral vein and positioned just below the renal vessels. In the appropriate position, the filter prevents thrombus from embolizing to the pulmonary vasculature. Long-term patency rate of the filter has been shown to be 98% in several larger studies.13 Recurrence of PE after an IVC filter is approximately 3%, while death from recurrent PE is less than 1% in those with IVC filters in place.65
However, the filters do not stop the thrombotic process.46 Patients may develop collateral flow around the filter. Rarely, caval thrombosis may occur with massive edema of both legs. IVC filters have been used as prophylaxis in those at high risk of bleeding or in those with chronic lung disease who may not tolerate a PE. In one study, IVC filters and anticoagulation were no better than anticoagulation alone in decreasing the two-year mortality rate. Any benefit for prevention of PE was counterbalanced by recurrent DVT.76
Pulmonary Embolectomy
Massive PE can be devastating even with appropriate treatment. For patients with massive PE who remain hemodynamically unstable despite appropriate resuscitative efforts, pulmonary embolectomy may be the only option. The indications for this procedure are shock despite heparin and pressors, failure of thrombolytic therapy, or contraindication to thrombolytics.13 Operative mortality with cardiopulmonary bypass ranges from 10-75% in various uncontrolled case series. More recent advances of thrombus extraction or fragmentation through a transvenous catheter system show promise; however, the mortality rate remains high at 27%.13
Calf Vein Thrombosis
This is a special situation that commonly is encountered in the ED. In the past, calf vein thrombosis was treated with rest, elevation, and a nonsteroidal anti-inflammatory agent. However, the risk is that the thrombus will propagate into the thigh and possibly embolize. In patients who are symptomatic, the current recommendation is to treat with anticoagulation for at least three months. Another option is to follow the patient with serial ultrasound in 7-10 days looking for thrombus progression.47
Conclusion
The treatment of DVT and PE requires systematic assessment and patient evaluation, frequently with multiple diagnostic modalities. Treatment options range widely from inpatient and/or outpatient therapy with LMWHs such as enoxaparin to more traditional approaches with UFH. Final decisions regarding outcome-effective therapy should be based on sound clinical judgment, risk stratification, and the patient’s ability to accept self-administration of parenteral anticoagulation therapy.
References
1. Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group (MEDENOX). N Engl J Med 1999;341: 793-800.
2. Alikhan R, Cohen A, Combe S, et al. Benefit of enoxaparin in medical patients: A subgroup analysis. www.abstracts-on-line.com, March 1, 2002, No. 1118.
3. Nicolaides AM. Prevention of venous thromboembolism: European Consensus Statement. Int Angiol 1992;11:151-159.
4. Clagett GP, Anderson FA, Geerts WH, et al. Prevention of venous thromboembolism. Chest 1998;114:1S-560S.
5. Prevention of venous thromboembolism: International consensus statement (guidelines according to clinical evidence). Int Angiol 1997;16: 3-38.
6. Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: A meta-analysis of randomized clinical trials. Thromb Haemost 2000;83:14-19.
7. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001;119:132S-175S.
8. Medicare Audits, Ltd. Disease Management Database 2000. Medicare Audits Ltd., UK.
9. Fagot JP, Flahault A, Kanfer A, et al. Prevention of venous thromboembolism in patients hospitalized for more than 24 hours in a medical ward: Proposed indications for low molecular weight heparins. Presse Medicale 2000;29:4-10.
10. Turpie AGG. Management of venous thromboembolism: Optimization by clinical trials. Haemostasis 1996;26(4):220-226.
11. Dalen JE, Albert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis 1975;17:257-270.
12. Heit JA, Silverstein MD, Mohr DN, et al. Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch Intern Med 1999;159:445-453.
13. Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic Therapy for Venous Thromboembolic Disease. Chest 2001;119:176S-193S.
14. Raschke RA, Reilly BM, Guidry JR, et al. The weight-based heparin dosing nomogram compared with a "standard care" nomogram. A randomized controlled trial. Ann Intern Med 1993;119: 874-881.
15. Levine M, Gent M, Hirsh J, et al. A comparison of low molecular weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677-681.
16. Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thromboembolism with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low molecular weight heparin administered at home. N Engl J Med 1996;334: 682-687.
17. Fiessinger JN, Lopez-Fernandez M, Gatterer E, et al. Once-daily subcutaneous dalteparin, a low molecular weight heparin, for the initial treatment of acute deep vein thrombosis. Thromb Haemost 1996;76:195-199.
18. Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med 1992; 326:975-982.
19. Hirsh J, Warkentin TE, Raschke R, et al. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 1998; 114:489S-510S.
20. Samama MM, Bara L, Gouin-Thibault I, et al. New data on the pharmacology of heparin and low molecular weight heparins. Drugs 1996;52(Suppl 7):8-15.
21. Thompson-Ford JK. Low-molecular-weight heparin for the treatment of deep vein thrombosis. Pharmacotherapy 1998;18(4): 748-758.
22. Vinson DR, Berman DA. Outpatient treatment of deep venous thrombosis: A clinical care pathway managed by the emergency department. Ann Emerg Med 2001;37:251-258.
23. Planes A, Vochelle N, Darmon JY, et al. Efficacy and safety of postdischarge administration of enoxaparin in the prevention of deep venous thrombosis after total hip replacement: A prospective randomised double-blind placebo-controlled trial. Drugs 1996;52(Suppl 7):47-54.
24. Borris LC, Lassen MR. Thromboprophylaxis with low molecular weight heparin after major orthopaedic surgery is cost effective. Drugs 1996; 52(Suppl 7):42-46.
25. Clagett GP, Anderson FA Jr, Geerts W, et al. Prevention of venous thromboembolism. Chest 1998; 114:531S-560S.
26. Leizorovicz A. Comparison of the efficacy and safety of low molecular weight heparins and unfractionated heparin in the initial treatment of deep venous thrombosis: An updated meta-analysis. Drugs 1996;52 (Suppl 7):30-37.
27. Brewer D. Should low-molecular-weight heparins replace unfractionated heparin as the agent of choice for adults with deep venous thrombosis? J Fam Pract 1998;47:185-192.
28. Spiro TE. A multicenter clinical trial comparing once and twice-daily subcutaneous enoxaparin and intravenous heparin for the treatment of acute deep vein thrombosis. Blood 1997;90(Suppl 1):295A.
29. Schoenenberger RA, Pearson SD, Goldhaber SZ, et al. Variation in the management of deep vein thrombosis: Implications for the potential impact of a critical pathway. Amer J Med 1996;100: 278-282.
30. Ebell MH. Low molecular weight heparins for DVT. J Fam Pract 1994;39:501-502.
31. Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med 1992;326: 975.
32. de Valk HW, Banga JD, Wester JW, et al. Comparing subcutaneous danaparoid with intravenous unfractionated heparin for the treatment of venous thromboembolism. A randomized controlled trial. Ann Int Med 1995;123:1-9.
33. Leizorovicz A, Simonneau G, Decousus H, et al. Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: A meta-analysis. BMJ 1994;309:299-304.
34. Lensing AW, Prins MH, Davidson BL, et al. Treatment of deep venous thrombosis with low-molecular-weight heparins. A meta-analysis. Arch Intern Med. 1995;155:601-607.
35. Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330-1335.
36. Simonneau G, Charbonnier B, Decousus H, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous unfractionated heparin in the treatment of proximal deep vein thrombosis. Arch Intern Med 1993;153:1541-1546.
37. Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996;334:677-681.
38. Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 1996;334:682-687.
39. Schafer AI. Low-molecular-weight heparin—An opportunity for home treatment of venous thrombosis. N Engl J Med 1996;334: 724-725.
40. Hirsh J, Poller L, Deykin D, et al. Optimal therapeutic range for oral anticoagulants. Chest 1989;95(sup 2):5S.
41. Bolan CD, Alving BM. Recurrent venous thrombosis in hypercoagulable states. Am Fam Physician 1991;44:1741.
42. Humphries JE. Acquired antithrombin-3 deficiency replacement with antithrombin-3 concentrates in a patient with protein S deficiency accelerates response to therapy. Acta Hematologica 1993; 90:151-154.
43. Anon. Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992;340:873-876.
44. Mohiuddin SM, Hilleman DE, Destache CJ, et al. Efficacy and safety of early versus late initiation of warfarin during heparin therapy in acute thromboembolism. Am Heart J 1992;123:729-732.
45. Hull RD, Raskob GE, Rosenbloom D, et al. Heparin for five days as compared with 10 days in the initial treatment of proximal venous thrombosis. N Engl J Med 1990;322:1260-1264.
46. Goldhaber SZ. Pulmonary Embolism. N Engl J Med 1998;339: 93-104.
47. Hyers TM. Venous Thromboembolism. Am J Respir Crit Care Med 1999;159:1-14.
48. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and Low-Molecular-Weight Heparin. Chest 2001;119:64S-94S.
49. Prandoni P. Heparins and Venous Thromboembolism: Current Practice and Future Directions. Thromb Haemost 2001;86:488-98.
50. Ageno W, Huisman MV. Low-Moleclar-Weight Heparins in the Treatment of Venous Thromboembolism. Curr Control Trials Cardiovasc Med 2000;1:102-105.
51. Hirsh J, Anand SS, Halperin JL, Fuster V: Guide to Anticoagulant Therapy: Heparin. Circulation 2001;103:2994-3018.
52. Bauer KA. The Thrombophilias: Well-defined risk Factors with Uncertain Therapeutic Implications. Ann Intern Med 2001;135: 367-373.
53. Hovanessian HC. New-Generation Anticoagulants: The Low Molecular Weight Heparins. Ann Emerg Med 1999;34:768-779.
54. Merli G, Spiro TE, Olsson CG, et al. Subcutaneous Enoxaparin Once or Twice Daily Compared with Intravenous Unfractionated Heparin for Treatment of Venous Thromboembolic Disease. Ann Int Med 2001;134:191-202.
55. Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin vs. heparin in the treatment of patients with pulmonary embolism. Arch Inter Med 2000;160:229-236.
56. Breddin HK, Hach-Wunderle V, Nakov R, et al. Effects of a low-molecular-weight heparin on thrombus regression and recurrent thromboembolism in patients with deep-vein thrombosis. N Engl J Med 2001;344:626-631.
57. The Columbus Investigators. Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med 1997;337:657-662.
58. Dolovich LR, Ginsberg JS, Douketis JD, et al. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism. Arch Intern Med 2000;160:181-188.
59. Van den Belt AGM, Bossuyt PMM, Prins MH, et al. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis—An economic evaluation. Thrombo Haemost 1998; 79:259-263.
60. Chong BH. Heparin induced thrombocytopenia. Br J Hematol 1995;89:431-439.
61. Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330-1335.
62. Bergqvist D, Benoni G, Bjorgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med 1996;335: 696-700.
63. Weitz JI. Low-molecular-weight heparins. N Engl J Med 1997; 337:688-698.
64. Hirsh J, Dalen JE, Deyken D, et al. Heparin: Mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy and safety. Chest 1992;104:337S-351S.
65. Comess KA, DeRook FA, Russell ML, et al. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000;109:351-356.
66. Tanios MA, Simon AR, Hassourn PM. Management of venous thromboembolic disease in the chronically critically ill patient. Clinic Chest Med 2001;32:105-122.
67. Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism. Chest 1999;115:1695-1707.
68. Meneveau N, Schiele F, Metz D, et al. Comparative efficacy of a two-hour regimen of streptokinase versus alteplase in acute massive pulmonary embolism: Immediate clinical and hemodynamic outcome and one-year follow-up. J Am Coll Cardiol 1998;31:1057-63.
69. Wells PS, Forster AJ. Thrombolysis in deep vein thrombosis: Is there still an indication? Thromb Haemost 2001;86:499-508.
70. Goldhaber SZ. Thrombolysis in pulmonary embolism: A debatable indication. Thromb Haemost 2001;86:444-451.
71. Hamel E, Pacouret G, Vincentelli D, et al. Thrombolysis or heparin therapy in massive pulmonary embolism with right ventricular dilation. Chest 2001;120:120-125.
72. Greenfield LJ, Michna BA. Twelve-year clinical experience with the Greenfield vena caval filter. Surgery 1988;104:706.
73. Fink JA, Jones BT. The Greenfield filter as the primary means of therapy in venous thromboembolic disease. Surg Gynecol Obstet 1991;172:253.
74. Cohen JR, Grella L, Citron M. Greenfield filter instead of heparin as primary treatment for deep venous thrombosis or pulmonary embolism in patients with cancer. Cancer 1992;70:1993-1996.
75. Ferris EJ, McCowan TC, Carver DK, et al. Percutaneous inferior vena caval filters: Follow-up of seven designs in 320 patients. Radiology 1993;188:851-856.
76. Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med 1998;338: 409-415.
