CDC finishing IC guidelines for smallpox vaccinees
CDC finishing IC guidelines for smallpox vaccinees
People will read this and hang on every comma’
Struggling mightily to balance the limits of science against the demand for clinical guidance, infection control experts are hammering out infection control guidelines for vaccinia virus in health care settings.
According to a draft of the guidelines obtained by Hospital Infection Control, the Centers for Disease Control and Prevention (CDC) will emphasize hand hygiene and standard barrier precautions.
The guidelines add contact precautions for treating certain adverse reactions following smallpox immunization. (See chart below)
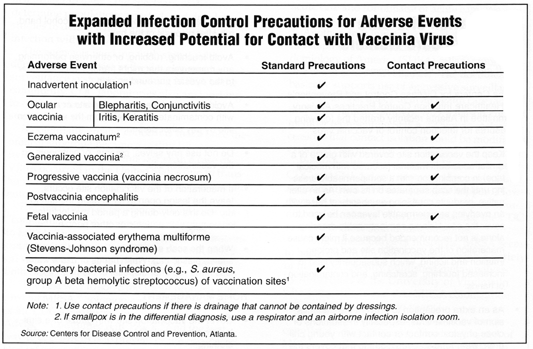
Developed by the CDC’s Healthcare Infection Control Practices Advisory Committee (HICPAC), the Infection Control Guidelines for Prevention of Transmission of Vaccinia after Smallpox Vaccination are designed for the current pre-attack stage in which some 400,000 health care workers are expected to be immunized.
The guidelines call for wearing gloves and gowns, if needed, for contact with body sites or materials and items that may be contaminated with vaccinia virus. Hand hygiene should be performed after manipulating the vaccination site or handling any items potentially contaminated with vaccinia virus — even when gloves are worn.
When hands are not visibly soiled, use either antimicrobial soap and water or an alcohol-based hand rub. When hands are visibly soiled, wash hands with antimicrobial soap and water, HICPAC recommends.
In addressing environmental issues, the panel recommended bagging and disposing of contaminated dressings and other materials in accordance with local regulations for medical waste. In home settings, place contaminated dressings in a sealed plastic bag prior to discarding with other household waste.
Contain vaccinia-contaminated materials without shaking, the guidelines advise. Promptly launder clothing, bedding, and other textiles that may be contaminated with vaccinia virus in a warm water cycle followed by hot-air drying. The additional of bleach (chlorine or nonchlorine) is not necessary for purposes of disinfection but will not interfere the virucidal activity of detergent and hot water; therefore, bleach may be added if desired.
Disinfect environmental surfaces and medical instruments that may have been contaminated with vaccinia virus according to standard procedures. Disinfectants commonly used in health care and household settings (e.g., Lysol) are appropriate, if used in accordance with manufacturers’ recommendations.
The world is watching
At a recent HICPAC meeting to finalize a draft version of the guidelines, panel members were acutely aware of the importance of the document as they dissected every detail. "The world is watching us," said Loretta Fauerbach, MS, CIC, a liaison member of HICPAC and the director of infection control at Shands Hospital at the University of Florida in Gainesville.
As a result, the committee was often mired in minutia, trying to determine whether, for example, health care workers who anticipate no patient contact can simply wear a gauze over the vaccinated site rather than the semipermeable dressing. The draft version, which is subject to revision, recommends that those with "no patient or health care contact" should cover the vaccination site loosely with gauze or a similar absorbent material, secured with medical tape, until the scab separates on its own. If no contact with other people is anticipated, the site may be left uncovered.
"I don’t think it is [that] hard; I just don’t see patients," said James Steinberg, MD, a liaison HICPAC member representing the Society for Healthcare Epidemiology of America. Yet others noted that unanticipated contacts with patients and colleagues can occur easily even if the recently immunized worker does not plan to see patients. A driving factor appears to be liability concerns by hospital attorneys, who want maximum "take" site coverage to ensure the vaccinia virus is not transmitted to patients or colleagues.
"I think this is a legal issue," Fauerbach said. "Many of the lawyers say you must use maximum precautions within your facility especially if you have [a vulnerable patient population]."
The gauze/dressing discussion arose in part because two members of the committee — Fauerbach and Alfred DeMaria Jr., MD, state epidemiologist in Boston — reported that they had adverse skin reactions to the adhesive on the semipermeable dressings after they were immunized for smallpox. "What’s happening in health care facilities is — at least in Massachusetts — is there is no way [vaccinees] can even visit those facilities without wearing the [semipermeable membrane dressing]," DeMaria said.
Steinberg responded, "I think we are overusing it, and I think that is part of the problem."
While the committee added language warning about sensitivity to bandage adhesives, veteran epidemiologist and HICPAC member William Scheckler, MD, concluded that the reports from the two committee members were too serendipitous and anecdotal to form the basis for infection control policy.
"I don’t know if it is a real rate," said Scheckler, hospital epidemiologist at St. Mary’s Hospital in Madison, WI. "It is certainly a real reaction. Certainly, we have not seen that kind of reaction for IV patients [who are treated with semipermeable dressings.]" The committee plans to include reactions to the adhesive bandaging in the adverse reporting system in order to determine if the problem could be widespread.
Scheckler warned that the draft version also has an apparent "disconnect" that urges workers to keep the site bandage intact at work but allows them to remove it at home.
"We recommend to change it every three to five days or when it is juicy’ [saturated]," he said. "This [draft guideline] sort of makes it look like you can take it off at home and change it every day. It seems to me that is a disconnect. I certainly have tried to sell this [to hospital workers] as the bandage coverage protects folks at home as well as it protects patients."
The smallpox vaccination programs should include a site-monitoring component to assess the dressings of all vaccinated health care workers daily, determine if dressings need to be changed, change dressings if indicated, and reinforce education about the need for meticulous hand hygiene. Only vaccinated staff should be designed to perform site care management (e.g., dressing changes or other manipulations of the vaccination site). However, the committee agreed that unvaccinated staff may perform visual inspections of the site that do not require removal or manipulation of the dressing.
Still, Scheckler argued that the guidelines should emphasize that workers should leave the dressing intact when at home. "If it needs to be changed, preferably, it should be changed by the site person at work," he stressed.
DeMaria advised a more permissive policy, noting that the military experience with smallpox vaccine shows that it is "no big deal" to take the bandage off at home.
"It’s a big deal for our lawyers," Scheckler replied.
HICPAC chairman Robert Weinstein, MD, tried to defuse the debate, saying, "We’re just saying that if they don’t want to wear it at home, they don’t have to."
The document cannot direct clinical practice down to every detail, added committee member Jane Siegel, MD, professor of pediatrics at the University of Texas Southwestern Medical Center in Dallas. "I think that it is up to people locally how they are going to operationalize this."
The problem with that position is that there is a perception that clinicians are desperate for direction regarding a potentially dangerous vaccine. They are looking to the CDC to make the close calls on the infection control aspects of vaccinia.
"Such extraordinary care has gone into the development of this program that people will read this and hang on every comma," said Michael Taper, MD, committee member and epidemiologist at Lenox Hill Hospital in New York City. "[They] are looking for absolutely the right way to do it. There is almost a sense that people really don’t want discretion. They want to be told the correct way to do this because there has been — some we even say excessive — concern about the risks."
On the other hand, attempts to draw conclusions and shape medical practice about many aspects of smallpox vaccine may push the guidelines beyond the currently known science, warned Marjorie Underwood, RN, BSN, CIC, committee member and infection control coordinator at Mt. Diablo Medical Center in Concord, CA.
"We have to be careful here," she told the committee. "We somehow have to strike a balance. There is this hysteria developing because of the initial messages of concern. Again, [we must] go back to what we know the science is. I know we would like to do more, but I think these documents should [reflect scientific data]."
Plans call for the guideline to be published by the CDC and possibly posted sooner in draft version on its smallpox web site (www.bt.cdc.gov/). The latter possibility hinges on approval of top CDC officials who are running the smallpox plan.
"There is a great deal of interest," said Steve Solomon, MD, acting director of the CDC division of healthcare quality promotion.
"[Our goal] is to get this information out into the hands of the infection control community as quickly as it is possible to do so. As soon as HICPAC has a version that it feels is ready to be posted, we will take that up the line [for approval]," he explained.
