Chemical Warfare Agents: Part II - Nerve Agents, Blood Agents, and Protective Gear
Part II: Nerve Agents, Blood Agents, and Protective Gear
Author: Charles E. Stewart, MD, FACEP, Emergency Physician, Colorado Springs, CO.
Peer Reviewers: Cynthia K. Aaron, MD, FACMT, FACEP, Associate Professor of Emergency Medicine; Director, Clinical Toxicology Services, University of Massachusetts Memorial Medical Center, Worcester, MA; Jonathan L. Burstein, MD, FACEP, Director of Disaster Medicine, Beth Israel Deaconess Medical Center; Assistant Professor of Medicine, Harvard Medical School, Boston, MA.
This issue is the second and final part in a series on chemical warfare agents. Part I focused on choking agents, vesicants, and halogenated oximes. This article will cover nerve agents, blood agents, and protective gear.—The Editor
Nerve Agents
Nerve agents in current use, storage, or production include tabun (GA), sarin (GB), soman (GD) ("G" for German—found in German military stores after World War II), and VX. The first three agents, the so-called "G" agents, are highly toxic organophosphate compounds that were developed between World War I and World War II. Although the Nazis had these agents during World War II, they never were used.
Another, more persistent nerve agent, code-named VX, was discovered by the British chemist R. Ghosh soon after World War II.1 Since the discovery of VX, there have been only minor developments in the toxic science of lethal chemicals.
More recently, five containers of the nerve agent sarin were placed on three Tokyo subway lines in 1995. In all, 5000-6000 people were exposed, 3227 were evaluated in emergency departments (EDs), 493 were admitted to hospitals, and 12 people died as a result of this terrorist exposure.2,3 The concentration in the rail cars with the containers was high, but the agent was not spread well through the subway system, and most exposures were mild. Of the ambulance personnel taking care of these victims, 135 developed symptoms and 33 were hospitalized. Many of the hospital staff also required treatment. Neither ambulance personnel nor hospital staff had any protection.
The vapor pressures of the "G" agents make them significant inhalation hazards in any warm climate or with droplet aerosols. VX is an oily liquid that persists on scene for weeks or longer. It is considered a "persistent nerve agent." All four of the agents may be well-absorbed through the skin. Nerve agents irreversibly inhibit acetylcholinesterase.
These agents combine with acetylcholine to prevent the synaptic transmitter functions of acetylcholine at the neuroreceptor junction.4 Acetylcholine will accumulate at all cholinergic junctions and cause a loss of function at those junctions. This explains the peripheral and central effects of these agents, but no direct evidence exists unequivocally relating the nerve agent toxicity solely to acetylcholinesterase inhibition.5
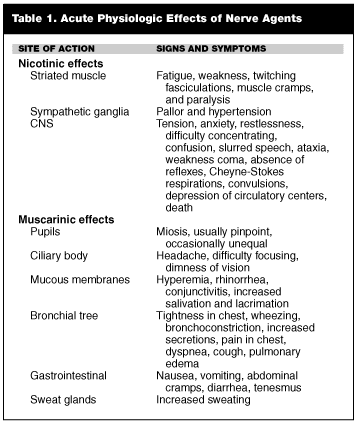
The importance of nerve agents’ peripheral effects is paramount. These include the stimulation of the endings of the parasympathetic nerves at the smooth muscle of the iris, the ciliary body, the bronchial tree, gastrointestinal tract, bladder, and blood vessel.6 This includes the activation of secretory glands of the respiratory tract and stimulation of the cardiac muscle. Sympathetic stimulation includes nerves to the sweat glands. These are the characteristic muscarine-like signs and symptoms associated with cholinergic excess (muscarinic effects).
Salivation, lacrimation, and defecation result from these effects of the nerve agent or muscarinic end organs. Bronchoconstriction, laryngospasm, and airway obstruction likewise may contribute to the lethality of the agents from the muscarinic effects.
The accumulation of acetylcholine at the endings of the motor nerves to voluntary muscles and the autonomic ganglia results in nicotinic-like signs and symptoms. For muscle, this would mean the following sequence of events would occur:
- Spontaneous activation of myofibrils (fasciculations) as acetylcholine accumulates and paralyzes random neuromuscular junctions;
- Tremors and twitching as entire muscle groups are lost; and
- Flaccid paralysis as the entire muscle group loses the effective function of the neuromuscular junction.
Finally, the accumulation of excessive acetylcholine in the brain and spinal cord is thought to result in central nervous system symptoms of twitching, jerking, staggering gait, convulsions, and coma. Objective changes in the electroencephalogram may be demonstrated.7
Individuals poisoned by a nerve agent display similar symptoms regardless of exposure route. The intensity and sequence of the symptoms, however, is influenced by the route of absorption. Symptoms include:
- Rhinorrhea
- Bronchial secretions
- Tightness of the chest
- Bronchospasm
- Dimness of vision
- Eye pain (in vapor or ocular exposure)
- Miosis
- Dyspnea
- Drooling and excessive sweating
- Nausea
- Vomiting, cramps, and involuntary defecation and urination
- Twitching, jerking, and staggering gait
- Headache, confusion, coma, and convulsions
- Respiratory depression and respiratory arrest
Skin exposure may produce localized sweating and fasciculations as the first effect. Eye exposure may produce eye pain, miosis, and dimness of vision as first effects.
Other common effects and complications include hypoxia, ischemia, acidosis, hyperthermia, hypothermia, peripheral neuropathy, and cerebral edema. These have been seen in patients who have received convulsive doses of nerve agents.8 It is obvious that many of the longer-term effects are related directly to the problems of providing adequate ventilation to the contaminated, seizing patient who has copious airway secretions and is not breathing spontaneously.
A summary of organophosphate-induced muscarinic and nicotinic symptoms is shown in Table 1.
Nerve agents may be delivered in vapor, droplet, or a combination of both forms. Any artillery or mortar capable of delivering a chemical munition is suitable. M55 rockets and M23 land mines are two such munitions. Low-flying missiles or aircraft that deliver a droplet spray are ideal. The Soviets have adapted Scud missiles to "splatter" these agents with a small explosive charge. Finally, release of the vapor or droplets from pressurized containers in aerosol forms can be accomplished. Delivery patterns will be dependent on the munition, capacity of the chemical container, and weather patterns.
Symptoms appear much more slowly from absorption through the skin than from respiratory exposure. Although the skin absorption of a lethal dose may occur within 1-2 minutes, it may take up to 1-2 hours for death to occur. If inhaled or absorbed through eyes or mucous membranes, the agent kills in 1-10 minutes. If food or water contaminated with nerve agents is ingested, the victim may experience symptoms in about 30 minutes.
When systemic symptoms are produced, no matter by what route of exposure, the red blood cell and/or plasma cholinesterase activity will be depressed, usually to below 30% of baseline levels. As a guide, in the absence of other causes, a cholinesterase activity of less than 50% of baseline would indicate exposure to an anticholinesterase agent. This measurement is not readily available in the field.
The most common cause of death after acute exposure to nerve agents is respiratory arrest, originally thought to be due to the flaccid paralysis of the respiratory muscles. Respiratory arrest often will occur prior to neuromuscular blockade and is not always due to muscle paralysis. Multiple animal studies have shown that the respiratory depression occurs before the neuromuscular blockade and the bronchoconstriction have reached significant proportions.9 These studies support the contention that a major contribution to respiratory failure is central nervous system toxicity rather peripheral toxicity.
Fortunately, the need for ventilatory support and intensive care for sarin casualties treated in Tokyo was only 24-48 hours when both atropine and an oxime were given.3 Even patients with severe signs of poisoning recovered completely if adequate supportive therapy and antidotes were given early.
Nerve agents also result in a number of delayed toxicities. The inhibition of cholinesterase enzymes is irreversible unless rapidly reactivated by an appropriate oxime, so effects are prolonged. Until the tissue cholinesterase enzymes are restored to normal levels, there is a period of increased susceptibility to another exposure of any nerve agent. This regeneration of enzyme levels may take as long as 2-3 months.
During this period of enzyme regeneration, the effects of repeated exposures are cumulative. Among these delayed effects are sudden cardiac failure in patients who apparently have recovered from the effects of organophosphate exposure.10
Organophosphate exposure can produce both central and peripheral nervous system signs and symptoms if the patient survives the respiratory failure and other potentially immediately lethal events. The symptoms may include impaired memory, hallucinations, fatigue, confusion, and concentration deficits.11,12 Signs may include both central and peripheral neuropathies and late seizures.
There is animal evidence that prolonged nerve agent-induced toxic convulsions produce irreversible brain damage. Benzodiazepine anticonvulsants appear to reduce the morbidity associated with these convulsions and also may protect the brain from significant toxicity.
Recent work has proposed that the sole toxic effect of the nerve agents is not just the inactivation of acetylcholinesterase. One study has described agonistic effects at the nicotinic cholinesterase receptor sites from tabun, sarin, and soman.13 These agents are capable of activating the ionic channel in a manner similar to acetylcholine. The study also noted that VX prevents ionic conductance through the channel, even if acetylcholine binds to the nicotinic receptor site. This means that there is no single site of treatment for an antidote.
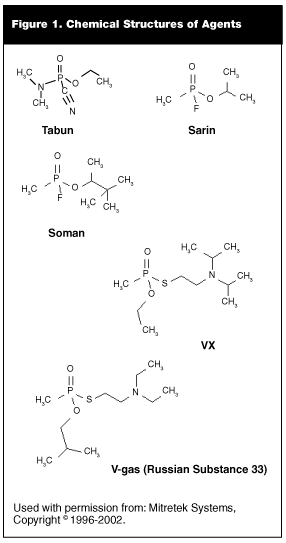
Tabun. Tabun or GA is ethyl N,N-dimethylphosphoroamidocyanidate. (See Figure 1.) It has a faintly fruity odor and is the first-discovered anticholinesterase nerve agent. It is the least toxic of the nerve agents. The median lethal dose is about 200 mg-min/m3 via inhalation, and about 20,000-40,000 mg-min/m3 via percutaneous absorption.
Tabun has a low volatility, but does evaporate from skin. Tabun would be expected to present both vapor and liquid phase contamination on victims exposed to this agent. Tabun has a half-life in the environment of about one to one and a half days. It is effectively detoxified on humans with bleach or soapy water. Equipment may be detoxified effectively with a dilute alkali solution, steam and ammonia, or bleach solution.
Although tabun is considered to be out of date and of limited use by military authorities, it is the easiest of the nerve agents to manufacture. As such, it is quite likely to be used as a terrorist agent.
Sarin. Sarin, or GB, is isopropyl methylphosphonofluoridate. (See Figure 1.) It has no odor or color when pure. It is classified as an organophosphate anticholinesterase.
The medical lethal dose of sarin (LCt50) is between 70 and 100 mg-min/m3 for inhalation and about 12,000-15,000 mg-min/m3 for percutaneous absorption. Sarin may persist for as long as five days.
Sarin is very volatile (22,000 mg/m3 at 25° C) and is absorbed mainly by respiration. The vapor pressures of sarin make it a significant inhalation hazard in any warm climate or with droplet aerosols. Indeed, sarin has a volatility that is close to that of water and is the most volatile of the nerve agents. It rapidly evaporates from the skin, thus limiting dermal exposure. The vapor is heavier than air, so it flows into low-lying areas. These characteristics of sarin make it truly a "nerve gas."
Although cutaneous absorption has a higher dose, this is due to the low vapor pressure of sarin. If the evaporation is occluded, the LD50 for sarin is nearly identical to VX.
Sarin also is very soluble in water, so decontamination by water irrigation is effective. Sarin also is detoxified effectively on humans with bleach or soapy water. Equipment may be detoxified effectively with a dilute alkali solution, steam and ammonia, or bleach solution.
Because sarin evaporates so quickly, it requires little cleanup. This makes it desirable for a military that wants to disable or kill troops and then move equipment through the resulting destruction. An act of terrorism with sarin would leave little or no property damage and minimal clean-up costs. The volatility of sarin also makes it very easy to disseminate in closed spaces through ventilation, air conditioning, or heating ducts, thus increasing the desirability as a terrorist weapon.
The volatility of sarin gives it a significant vapor hazard for the medical provider during decontamination, but also makes it much less likely that the patient will have liquid contamination present. During decontamination of the patient contaminated with sarin, the medical provider must have adequate respiratory protection.2
Soman. Soman, GD, or pinacolyl methylphosphonofluoridate is a colorless liquid that has a fruity or camphor odor. (See Figure 1.) Its mechanism of action is similar to sarin, and it is made by the same process in the same equipment. The only difference is the alcohol used—pinacolyl alcohol instead of isopropyl alcohol.
Soman is the second most volatile of the nerve agents, with a volatility of 3900 mg/m3 at 25° C. By inhalation, the median lethal dose is 50-70 mg-min/m3 (LCt50), while absorption of only 100 mg is the median lethal percutaneous dose for a 70-kg man (LD50). The onset time of symptoms is about the same as sarin.
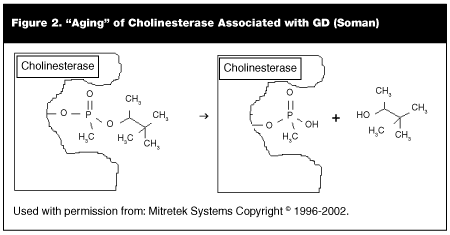
Soman "ages" quite rapidly when bound to the cholinesterase molecule (within 2 minutes.) (See Figure 2.) The phenomenon of aging (discussed in detail later) makes treatment of soman intoxication much more difficult. 2PAM (pralidoxime chloride) is not effective in reactivating cholinesterase bonded with soman. This makes soman the most difficult of the common agents to treat with antidote therapy.
Since soman and sarin both can be produced with the same equipment and with the same process, it is theoretically possible to mix both alcohols required and make a mixture of soman and sarin. This compound would have the volatility of sarin and the treatment difficulty of soman.
Soman is considered a relatively persistent agent, midway between sarin and tabun. It is less stable than tabun or sarin. The major route of action of soman is by dermal contact, not by inhalation, although there is some vapor production, particularly at higher temperatures. Soman can be thickened so that it is more persistent and has a volatility similar to VX. It is detoxified effectively on humans with bleach or soapy water. Equipment may be detoxified effectively with a dilute alkali solution, steam and ammonia, or bleach solution.
VX. The most toxic of the classic nerve agents is VX or O-ethyl S-(2-diisopropylaminoethyl) methylphosponothiolate. (See Figure 1.) It is more stable, less volatile, and more efficient at penetration of intact skin than the G agents. It is thought to be 10 times as toxic as sarin for humans by the percutaneous route. The median lethal dose by inhalation is about 30 mg-min/m3, while the percutaneous absorption makes the median lethal dose by the route as low as 6 mg-min/m3.
By inhalation, VX is about twice as toxic as sarin. Since VX is not very volatile, inhalation is not a major hazard with VX. V agents bind to acetylcholinesterase much more potently than the organophosphate and carbamate insecticides. The physiologic effects and onset times of symptoms are similar to sarin. VX is the slowest "aging" agent in common use. Patients exposed to VX may not have miosis. This is probably because the eye is not exposed directly to the agent, unlike with the vapor of the G agents. Miosis may be a delayed sign of VX exposure.
VX is an oily liquid that persists on scene for weeks or longer. Although not volatile enough to pose a major inhalation hazard, it is readily absorbed through the skin. Although VX is considered to have low volatility, vapor exposure is possible at temperatures greater than 100° F.
The other agents in the series are less known, and the information available about them mostly is classified. The other agents also have coded names, including VE, VG, and VM. V agents are approximately 10-fold more poisonous than sarin.
The former Soviet Union also produced several analogues to VX. The first was Substance 33, a compound similar to the persistent nerve gas VX, of which 15,000 tons were produced in the early 1980s. This agent often is called V-gas by U.S. writers. This nerve agent is thought to be similar in persistence and effect to the V agents.
V-gas (Russian substance 33). (All Soviet/Russian chemical warfare agents have their own code numbers similar to the United States two-letter designators. Sarin is coded by the Soviet system as Substance 35; soman is Substance 55.) (See Figure 1.) Two other similar unitary agent substances also were developed: A-230, which officially was approved by the Soviet Army in 1988, and A-232 (an agent similar to A-230) that was never formally approved.
The consistency of these Soviet agents is similar to oil; therefore, the inhalation hazard is less than with G agents. This consistency renders them toxic by dermal exposures. Since many of the agents remain classified, or information is not available in the open literature, this section discusses VX as the prototype of the series.
Most important to medical providers, VX is more difficult to decontaminate than the G agents. Since VX has a low volatility, the straw or clear liquid drops on the skin do not evaporate and will be absorbed more readily. It is a relatively persistent agent and lasts between two and six days in soil.
VX is only slowly hydrolyzed by water, and the hydrolysis products include EA2192, which is nearly as toxic as VX itself and longer lasting. Thus, hydrolysis-based decontamination schemes are not particularly effective against VX. Oxidation is the method of choice for VX decontamination. Common bleach (NaOC1-) and superchlorinated bleach (Ca (OC1-)2) react with VX at the N and S atoms and provide good decontamination.14-16
VX may be a much more attractive agent for the terrorist than sarin for several reasons. It is the most toxic of the nerve agents. When VX is dispersed, it is a persistent agent for weeks to months in the right climate. At least one of the degradation products is even longer lasting and just as toxic. This means that it is much more difficult and hence more expensive to clean up than the other agents. The Soviet Union produced large quantities of V-gas, a close analogue. This agent (or scientists who know how to manufacture it) may well be available for the right price to a terrorist.
Binary Agents. In 1908, the United States proposed production of a new variant of nerve agent—binary munition. The concept is to fill munitions with one of two agents, transport them to the battlefield, and load a second compartment in the projectile prior to use. When the projectile or bomb is fired or exploded, the two compartments rupture and the two agents combine. The product of the resulting reaction is a nerve gas such as sarin or VX. The munition is delivered in the usual fashion from mortar, plane, or artillery piece. The advantage is safety in transport and storage. There is no advantage in dissemination or decontamination. Many of these nerve agents are stored at sites around the United States.
General Treatment of Nerve Agents. The nerve agents have an extremely rapid effect. Treatment must be started quickly after initial symptoms or life support will be needed as respiratory arrest occurs. If multiple patients have severe exposure to a nerve agent, it is quite likely that some of them will die.
Triage of the patient should be based on the available resources, the type of exposure (liquid or vapor), and the need for decontamination before therapy is attempted. Based on the experience in Japan, those patients who have had exposure to nerve agent vapor and are still awake are not likely to get worse after arrival to the hospital.2 Decontamination should consist of removing all clothing and washing the hair to remove residual vapor.17
This is not true if the patient has liquid nerve agent exposure. Following exposure to nerve agent liquid, formal decontamination for the patient’s sake is a priority. Any residual nerve agent poses a continuing threat to the patient and to the responding medical staff.
Decontamination begins with removal of all clothing and irrigation with water. If the agent involved is VX, the decontamination team should be aware that hydrolysis converts VX into a much longer lasting and equally toxic metabolite. The patient’s hair should be scissor-trimmed close to the scalp, and the scalp should be washed with soap and water. After the irrigation and trim, the patient should be washed with soap and water or with bleach. (There is some controversy as to whether the decontamination team should dilute bleach used for decontamination of nerve agents. Since bleach generally is recognized as a safe substance, and even ingestions of small amounts of household bleach are considered innocuous, the author believes that 3% [household strength] hypochlorite solution is an acceptable decontamination solution. It has the advantages of ready availability and inexpensive.) The clinician should avoid razors, shears, stiff brushes, hot water, or scrubbing as all of these can abrade skin and enhance absorption of the nerve agent.18,19 Antidotes are not needed for asymptomatic patients.
Atropine. For more than 40 years, the standard therapy for emergency treatment of anticholinesterase agents has been atropine sulfate. Atropine relieves the symptoms, but does not attack the cause of the toxicity. Atropine protects against the excess of acetylcholine that occurs due to the inhibition of acetylcholinesterase by blocking the acetylcholine receptors.
Atropine only works in certain parts of the cholinergic nervous system. There are two types of acetylcholine receptors: the nicotinic, which are found in the skeletal muscles; and the muscarinic, which are found in the smooth muscles, exocrine glands, and the central nervous system. Atropine will reverse the muscarinic effects of nerve agent poisoning such as bronchospasm, excessive respiratory secretions, and intestinal hypermotility. Atropine provides no protection from the effects of the organophosphates on the nicotinic receptors at the muscular junctions.
Large doses of atropine may be required for reversal of the muscarinic effects of nerve agents. Although the military issues three autoinjectors with 2 mg of atropine each, this may be an inadequate dose for field treatment. Well-documented exposures requiring 20-40 mg of atropine are not unusual with exposure to the nerve agents. Doses of more than 2000 mg/day have been required in organophosphate pesticide poisonings.20 The discrepancy in atropine requirements may be due to the higher contamination load associated with oral ingestion of organophosphate pesticides vs. skin or vapor absorption of the nerve agents.
In the sarin release in the Tokyo subway, one report noted less use of atropine than was expected.2 This article noted that most patients had only limited exposure due to the ineffectiveness of the delivery system of the sarin. The article also pointed out that fully 82% of patients had only minimal aerosol exposure to the agent and felt that the decreased use of atropine was due to the decreased exposure. This also may be due to the decreased purity of the agent used.
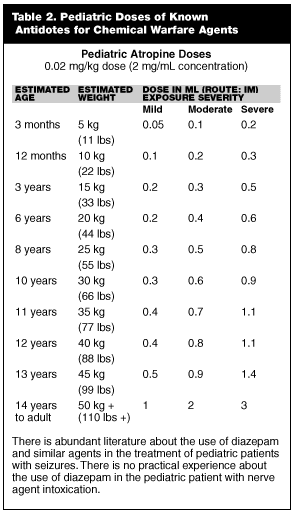
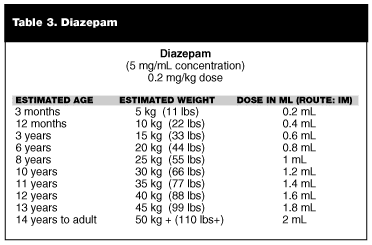
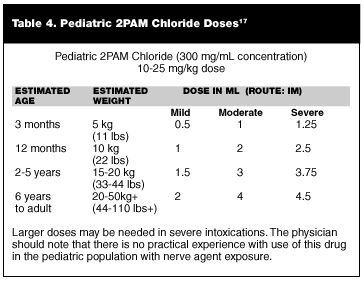
There is simply no available evidence regarding the use of atropine for treatment of nerve agent poisoning in the pediatric population. There is excellent experience with the use of atropine for other disorders and as a preoperative medication. There is some experience with pediatric overdoses on commercial organophosphates, but how this experience relates to nerve agent exposure is unknown. Doses have been extrapolated for pediatric populations based on weight, but there is no experimental or even good anecdotal data that validates these extrapolations. These extrapolations are given in Table 2.
Likewise, there is no evidence that supports (or refutes) a different dose of atropine in the geriatric population. The clinician quickly will realize that any listed "contraindication" to atropine is relative in the face of a surely lethal exposure to nerve agent.
Few ambulances are equipped with more than 10-15 mg of atropine within all of the drug boxes and resupplies carried on the ambulance. Likewise, few EDs have access to enough atropine to treat substantial numbers of severely affected victims. The ideal solution is rapid movement to the site of pre-positioned stocks of atropine, but this may not be available or practical. Additional sources of atropine include the operating room where preoperative atropine may be stored. Field expedient atropine sources include ophthalmic atropine and veterinary atropine.17
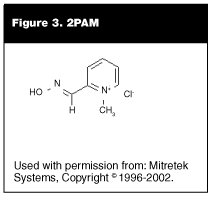
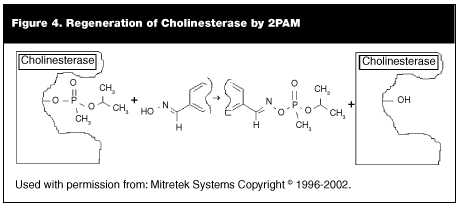
Pralidoxime (2PAM). In 1951, the oximes (pralidoxime) with the general formula of R-CH=NOH first were proposed as a treatment of poisoning by nerve agents. (See Figure 3.) These drugs are used to reactivate acetylcholinesterase bound by the nerve agent. (See Figure 4.) The oxime destroys the P-O bond in the nerve agent and a phosphorylated oxime is formed. This phosphorylated oxime rapidly is hydrolyzed to a non-toxic product. The efficiency of the oxime in reactivating acetylcholinesterase depends on the enzyme, the oxime used, and the nerve agent that is used. There is no single oxime available that is effective in reactivation of acetylcholinesterase that has been bound to all of the nerve agents.
Atropine and the oximes should be considered to complement each other, and the two antidotes appear to have a synergistic effect.
After a variable period (called the aging period), reactivation of the cholinesterase becomes very difficult.21 The nerve agent-inhibited enzyme undergoes a further chemical reaction, known as aging (shown in Figure 2 for soman). The alkyl ester group hydrolyzes to give the enzyme monoester. This reaction produces a particularly stable complex that is resistant to both hydrolysis and regeneration by oxime. After the aging reaction takes place, cholinesterase cannot be reactivated with oximes. Aging takes place in hours for sarin and VX, but occurs in about two minutes for soman; this makes soman exposure particularly difficult to treat with oximes. For this reason, VX and sarin are the easiest of the nerve agents to treat, and all oximes increase the chances of survival with these agents. For commercial organophosphates, aging occurs after 24-48 hours.
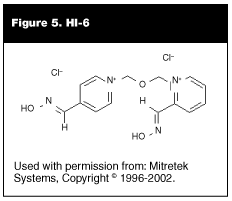
A subset of conventional oximes are the bispyridinium oximes or H oximes (H for Hagedorn). These include agents such as HI-6, HGG-12, and HGG-42. These oximes have been studied in the military setting but are not available for use in the United States. HI-6 is approved for use in other countries, but not yet in the United States. (See Figure 5.) H oximes have shown promise in reactivating aged enzyme after soman exposure.22 The bispyridinium oxime, obidoxime (Toxogonin) has been tested successfully for sarin and tabun intoxication. It is possible that HI-6 has some positive antidotal effects in addition to reactivation of the enzyme.23
Even self-administrated pralidoxime, given at the first symptoms of soman exposure, would not be completely effective treatment for soman poisoning due to the rapid aging phenomenon. Soman is the most difficult of the common nerve agents to treat and can be treated only with HI-6 due to this aging phenomenon.
Pralidoxime also is ineffective in treatment of tabun intoxication. Of all of the oximes, obidoxime and HI-6 appear to be the most effective antidotes against tabun poisoning.
Currently, the U.S. Army uses pralidoxime chloride to reactivate cholinesterase. It is not completely effective and is least effective against GD. VX, on the other hand, has an aging period of several hours and use of 2PAM is helpful in the treatment of this agent.
The U.S. military Mark I kit contains 2 IM autoinjectors, one with 2 mg of atropine and the other with 600 mg of pralidoxime, to be administered simultaneously in the event of nerve gas exposure. United States soldiers carry three MARK I kits. They are trained to administer one combination injector through protective clothing and undergarments if any symptoms of exposure occur. The recommended number of MARK I kits to be administered to a victim depends on the route of exposure, severity of clinical effects, and elapsed time after exposure.
Animal data suggest that a serum pralidoxime level of about 4 mcg/mL may be the minimal level to offer protection against toxic effects of organophosphates.24,25 Casualties in the Tokyo sarin release required as much as 36 mg of 2PAM.2 Endpoints for therapy include elimination of bronchorrhea (atropine) and improved muscle strength (oximes).
Continuous intravenous infusion of 2PAM for insecticidal organophosphate poisoning has been shown to be safe and effective. Current therapeutic guidelines suggest administration of 1 gram of pralidoxime chloride every 4-6 hours for organophosphate intoxication. Infusion (0.5 g/hr) provides serum levels of about 15 mcg/mL in steady-state simulation in adults and may provide better therapy in patients with organophosphate intoxication.26
In available case reports of nerve agent intoxication, however, severe toxic effects following the nerve agent exposure often last only a few hours, if the patient is thoroughly decontaminated and treatment with both atropine and 2PAM is begun early. In contrast to organophosphate insecticides, repeated pralidoxime dosing may not be necessary for nerve agent poisoning.
Pralidoxime rapidly is excreted in the urine, and the patient should have adequate hydration during therapy. Patients with renal failure will require lower doses for continued therapy. If intravenous access is difficult, a solution for intramuscular use can be made by mixing the contents of a 1-g vial with 3 mL of sterile saline. Intramuscular administration to a patient with an adequate blood pressure produces plasma concentration of 4 mg/L within 10 minutes.17
As noted above, oxime treatment of carbamate poisoning usually is not used. In cases where the medical provider does not know the nature of the agent, use of 2PAM will cause no harm to the patient and will protect the patient for organophosphate poisoning.
Diazepam and Other Benzodiazepines. If the patient presents with seizures or signs of severe intoxication, then diazepam should be administered in usual anticonvulsive doses. As noted earlier, the addition of diazepam to the treatment protocol is recommended because diazepam helps protect against seizures commonly found in nerve agent poisoning. Rapid treatment of seizures is essential to protect neurons.
In addition, diazepam has improved morbidity and mortality of soman poisoning independent of anticonvulsant effects.27 Diazepam appears to provide protection against some permanent damage that may result from heavy exposure to nerve agents.28
It is expected that all of the other benzodiazepines employed as usual antiseizure medications (i.e., lorazepam and midazolam) would be equally useful in this regard in the context of nerve agent therapy.
Pediatric Antidote Doses for Chemical Warfare Agents
Endpoints for therapy include elimination of bronchorrhea (atropine) and improved muscle strength (oximes). For all children weighing fewer than 70 pounds, the Mark I nerve agent antidote kit represents a serious overdose of atropine and 2PAM and risks vs. benefits should be considered quite carefully before it is used.
There is abundant literature about the use of atropine and similar agents as preoperative medications in the treatment of pediatric patients. There is limited experience with the use of atropine to treat organophosphate intoxication with commercial organophosphates. There is no practical experience about the use of atropine in the pediatric patient with nerve agent intoxication.
There also is abundant literature about the use of diazepam and similar agents in the treatment of pediatric patients with seizures. There is limited experience with the use of diazepam and similar agents to treat seizures due to organophosphate intoxication with commercial organophosphates. There is no practical experience about the use of diazepam in the pediatric patient with nerve agent intoxication.
Table 2 and Tables 3 and 4 below include suggested doses for 2PAM, atropine, and diazepam.
Pregnancy
The doses for each of the agents mentioned above are the same for the pregnant patient. Although 2PAM is a class C drug in pregnancy, there is simply no question that the choice between antidotes and death would lean heavily toward the use of 2PAM. Pregnant women have been treated successfully for commercial organophosphate poisoning with atropine and pralidoxime in the second and third trimesters of pregnancy and have delivered healthy newborns.
Blood Agents
Cyanide. Cyanide gas is famous as the lethal agent used for executions in many states. In high concentrations, it kills quickly. Cyanide was used in World War I, but did not prove to be as successful as chlorine, because of high volatility. The high volatility means that even high concentrations can’t be maintained for more than a few minutes in the open air.
Potassium cyanide poisoning of food and water supplies is an ancient terrorist tactic. It has been used in the past to contaminate drugs in the United States.29
Cyanide-containing compounds have been stocked by some nations for use as a chemical warfare agent. The military designators for the cyanide compounds used in warfare are:
- AC (hydrogen cyanide HCN)
- CK (cyanogen chloride CNCL)
The military incorrectly calls cyanide a "blood agent," implying that the action is in the blood when it is, in fact, a tissue toxin. The widespread distribution of absorbed nerve agents and vesicants through the blood makes this an antiquated term.
These agents may be delivered by munitions from artillery, mortar, bombs, or simply released from canisters. The preferred way to deliver cyanide is by large munitions because smaller weapons will not provide the concentration needed for lethal effect. As with all of the chemical warfare agents, area of action is weather- and wind-dependent.
Sources. Industry in the United States manufactures more than 300,000 tons of hydrogen cyanide each year. These cyanides are used in chemical processes, electroplating, mineral extraction, dye manufacture, printing, photography, and agriculture. Potential terrorists readily may find this agent in tank-car lots. Although it was not found to be particularly effective in World War I, the terrorist may find it more useful in a confined area or enclosed space.
Cyanide also is found in the fumes from burning x-ray film, wool, silk, nylon, paper, nitriles, rubber, urethanes, polyurethane, and other plastics. As a product of combustion, CN commonly is mixed with isocyanates, which are intense respiratory irritants. The reaction often is temperature-limited.
Cyanide appears to act by inhibition of the cytochrome oxidase system and subsequent interruption of the aerobic cellular metabolism. This leads to a profound lactic acidosis as the body attempts to use the less efficient anaerobic metabolism. Subsequent central nervous system (CNS), respiratory, and myocardial depression complicate the picture.
Cyanogen chloride has similar action to that of hydrogen cyanide. It is not lethal at lower concentrations, but it does possess potent pulmonary irritant and lacrimator effects.
Symptoms. In action novels, death from cyanide is so quick that there are few treatments available. It is not the surely lethal agent of the thrillers, however. Cyanide is the least toxic of the "lethal" chemical agents.
Cyanide is a significant exception to the Ct concept. Since the body has a mechanism for detoxification of cyanide, a long, slow exposure to cyanide has significantly different effects than the same amount of cyanide in a very short time.
Inhaled hydrogen cyanide can be quite lethal. Exposure to 140 ppm for 60 minutes or 1500 ppm for three minutes has an estimated 50% mortality. The inhaled dose at which 50% of the exposed people will die (LD50) is 0.5 mg/kg for hydrogen cyanide. Cyanide toxicity should be considered in all smoke-inhalation victims with CNS or cardiovascular findings.30,31
The symptoms are relatively nonspecific. Inhalation of cyanide agents may cause:
- Dryness and burning of the throat
- Air hunger
- Hyperpnea
- Apnea
- Seizures and coma
- Cardiovascular collapse
Early symptoms may include dryness and burning of the throat and air hunger. In small doses, headache, confusion, anxiety, dizziness, nausea, palpitations, tachycardia, tachypnea, and combativeness all may be found. In large doses, bradycardia, bradypnea, coma, gasping respirations, apnea, and death all may be common manifestations. Cyanosis generally is absent.
The examiner should suspect acute cyanide intoxication if the patient has had an abrupt collapse without apparent cause and subsequently does not respond well to oxygen administration. An audible gasp is thought to be characteristic of extreme exposure to HCN. This is followed in 15-30 seconds by the onset of convulsions. Respiratory activity stops in 2-3 minutes, and cardiac activity stops several minutes later.
Cyanogen chloride will cause irritation to the eyes, nose, and airway as seen with riot-control agents. The patient will develop marked lacrimation, rhinorrhea, and bronchial secretions. The later effects are similar to those seen with cyanide.
The success of therapy for acute cyanide intoxication depends primarily on the speed with which the cellular oxygen utilization is restored. The patient immediately should be removed from the contaminated atmosphere. Early use of a gas mask for the patient also will prevent further inhalation.
The diagnosis of hydrogen cyanide poisoning is difficult without a history of exposure. Particularly in the field, without laboratory support, these agents are difficult to identify. There are no specific physical findings that would implicate cyanide. The examiner may smell the odor of almonds, but about 50% of people are unable to smell cyanide’s odor.32
There is no readily available assay that can be done in real time to confirm cyanide poisoning while trying to treat an acutely poisoned patient. Spectrophotometry and gas chromatography are tools for the pathologist, not the clinician. A new semiquantitative assay that uses calorimetric test strips may improve the laboratory evaluation of hydrogen cyanide poisoning.33 Before any cyanide level is correlated with clinical appearance, the elapsed time since the exposure and since the specimen was obtained must be considered.
The Lee-Jones rapid cyanide diagnostic test may be performed on gastric aspirate but is not useful in inhalation injuries.
Lee Jones Test
- Add crystal FeSO4 to 5-10 cc of gastric contents.
- Add 4-5 drops of 20% NaOH.
- Boil and allow to cool.
- Add 8-10 drops of 10% HCL.
- A positive result for cyanide is a greenish-blue color.
- Salicylates will turn greenish-blue and then purple.
Arterial blood gases often will show a metabolic acidosis with normal oxygenation and calculated hemoglobin saturation. Venous gases have the same pattern, because the oxygen is not used up at the tissues. Venous blood often looks arterial in color. The measured arterial oxygen saturation will be decreased, while the calculated saturation is normal.34
This picture of an abnormal hemoglobin and less than adequate saturation is found commonly in only four poisons. The toxidrome includes cyanide, carbon monoxide, hydrogen sulfide, and methemoglobin. Methemoglobin and carboxyhemoglobin easily are measured. Hydrogen sulfide and cyanide are treated in a similar manner. Cyanide levels should be obtained in all cases. An elevated anion gap metabolic acidosis may exist but is not diagnostic. Lactic acid levels may be quite elevated in cyanide intoxication and may contribute substantially to the elevated anion gap acidosis, but this finding is nonspecific to cyanide.
Treatment. The definitive treatment of cyanide intoxication differs in various countries, but only one method is approved for use in the United States.
The mainstays of treatment are oxygen and ventilation. Contaminated clothing must be removed and the skin washed. Therapy beyond the basics is controversial and includes the cyanide antidote kit, hyperbaric oxygenation, and massive doses of vitamin B12. (The cyanide antidote kit is formerly the Pasadena kit, now made by Taylor Pharmaceuticals, which replaced the classic Lilly cyanide kit when Lilly stopped making it.)
There is evidence that cyanide intoxication responds to the administration of 100% oxygen. It should be a part of supportive care for all patients suspected of having cyanide intoxication.
There also is good evidence that some patients who have ceased breathing will survive when appropriate respiratory support is given. If the patient still has intact circulation, then airway support and antidote therapy may be life-saving. Lack of an antidote should not preclude treatment with airway support and ventilation in these patients.
Cyanide Antidote Kit. The United States military currently advocates the use of nitrites and thiosulfate for treatment of cyanide intoxication. Sodium nitrite (10 mL of 3% solution) is used intravenously followed by sodium thiosulfate (50 mL of 25% solution).
Proposed in the 1930s by Chen and colleagues, intentional production of methemoglobin is used to compete with cyanide for sites on the cytochrome oxidase system.35 Methemoglobinemia is produced by inhalation of amyl nitrite and then intravenous administration of sodium nitrite. About 30% methemoglobinemia is considered optimum, and the levels should be kept below 40%. Cyanide binds preferentially to the methemoglobin to form cyanmethemoglobin. After the methemoglobin has relieved the symptoms, the cyanide is converted to thiocyanate by the use of much less toxic sodium thiosulfate. The thiocyanate ion then allows renal excretion. A high tissue oxygen markedly potentates the effects of this treatment.
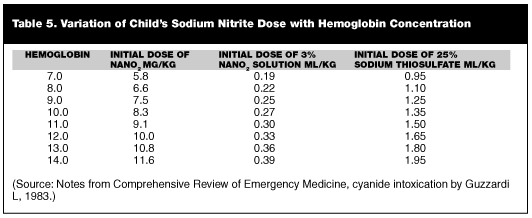
Therapy with nitrites is not innocuous, and the doses given to an adult can cause a fatal methemoglobinemia in children.36 (See Table 5.) In those with mixed gas exposure, induction of methemoglobinemia may induce tissue hypoxia. Administering the sodium nitrite too rapidly may cause vasodilation and hypotension. If methylene blue is given, all of the cyanide bound to the methemoglobin will be released with a relapse of symptoms. Instructions are on the cyanide kits and should be followed explicitly.
Hydroxycobalamin (Vitamin B12a). Hydroxycobalamin has been used to prevent cyanide toxicity from prolonged administration of sodium nitroprusside as well as in the acute treatment of cyanide poisoning.37-39 This agent reacts directly with the cyanide and does not act on the hemoglobin. Hydroxycobalamin appears to be a preferable antidote for patients with another concurrent gas exposure, such as carbon monoxide, and is used in France by prehospital providers for the treatment of smoke inhalation.40 There is limited use in the United States to date, but more than 15 years of experience are documented in the French literature.41 Hydroxycobalamin essentially is devoid of complications but is not currently available in the United States in the appropriate strength.
Another antidote available in Europe is dicobalt-EDTA, sold as Kelocyanor.42 This agent chelates cyanide as the cobalticyanide. This drug provides an antidote effect more quickly than formation of methemoglobin, but a clear superiority to methemoglobin formation has not been demonstrated. Dicobalt-EDTA causes a significant hypertension and may cause dysrrhythmias if no cyanide is present when it is given.
Kelocyanor and hydroxycobalamin may be given together for additive effect.43 One study evaluated 10 French patients who were given combinations of thiosulfate and hydroxycobalamin and felt that this combination had some additive effect.44
Hyperbaric Oxygenation. Hyperbaric oxygenation may be the ideal adjunct to both hydroxycobalamin and nitrite therapies. Hyperbaric oxygenation will mitigate concern about the methemoglobinemia formed by nitrite administration since the dissolved oxygen in the tissues and blood stream can support the metabolic requirements. The oxygen may act competitively to displace cyanide from the cytochrome oxidase.45 In the case of a mixed gas inhalation, carbon monoxide will be displaced effectively from hemoglobin and will allow higher levels of nitrite to be used. Hyperbaric oxygen therapy should not be used to replace the chemical treatments, however, due to the deleterious effects of delay in institution of the treatment in most cases. Hyperbaric oxygen therapy is unlikely to be available in the event of a terrorist attack with cyanide.
Arsine. Arsine is a highly toxic, colorless gas with a mild garlic-like odor. It is water-soluble and is absorbed readily by inhalation. Arsine is used widely as a "dopant" in the manufacture of semiconductors. Arsine also may be generated during smelting, welding, soldering, galvanizing, and refining.
Exposure to 250 parts per million (ppm) is rapidly lethal. Exposure to 25-50 ppm for 30 minutes can be fatal. Arsine binds to hemoglobin and causes hemolysis. It also is thought to liberate intracellular arsenic or bind to sulfhydryl groups essential for respiration.
Arsine is appropriately classified as a blood agent by the military. This agent actually causes significant hemolysis and subsequent kidney damage. Initial symptoms often begin 2-24 hours after inhalation. The initial symptoms may be headache, malaise, weakness, and dyspnea. The patient may complain of abdominal pain, nausea, and vomiting. These acute symptoms are followed in 4-6 hours by hematuria. Jaundice may be seen after 24 hours. The toxidrome of abdominal pain, hematuria, and jaundice is characteristic of arsine poisoning, though many patients will not have all three signs. Acute exposure to high concentrations of arsine may result in pulmonary edema.
Physical examination usually is unhelpful. The acute examination of the patient with significant toxicity may be indistinguishable from any of the pulmonary agents described later. Later in the course, the patient may have a yellow-bronze skin and hepatomegaly.
Laboratory examination shows a picture of hemolytic anemia with elevated plasma hemoglobins and hemoglobinuria. A history of exposure to arsine and a plasma hemoglobin of more than 1.5% would confirm the diagnosis of arsine poisoning. Arsenic levels in serum and urine are not helpful in management, but will corroborate the diagnosis.
Treatment includes exchange transfusion and renal dialysis. Hemolysis may continue for up to four days after dialysis since not all arsenic will be removed by dialysis. Death usually results from renal failure.
Improvisational Chemical Agents
Several factors limit the use of recognized chemical weapons by many terrorists, including the access to the chemicals used to manufacture the agents, difficulty and danger associated with production, and problems associated with dispersion. Simply stealing these agents from a government source involves breaching security surrounding the government chemical agent stockpiles and enhanced recognition that this particular chemical now presents a threat.
While there is no question that abundant stocks of these chemical weapons and/or ease of manufacture of the classic chemical weapons may favor their use by a terrorist organization, there is simply nothing in any rule book that mandates that a terrorist must use a recognized chemical warfare agent.
Any potent toxin could be used for incapacitation or sabotage. While the improvised chemical agent may be less toxic than military agents, the release of industrial chemicals at Bhopal, India, shows that the casualties resulting from an improvised chemical could be equally horrendous. At least 123 plants in the United States keep amounts of chemicals that, if released, could form toxic clouds that would put more than 1 million people in danger.46 More than 700 plants could put at least 100,000 people at risk, and more than 3000 facilities have at least 10,000 people nearby that would be affected by a release of the chemicals that are contained in the plant.
Many chemicals that might be used as improvised chemical weapons have been detailed in the CDC threat list. Other, more common industrial chemicals that could be used as terrorist weapons are included in Table 6.

It is likely that these chemicals will be deployed using available transportation such as tank cars, trucks, or even river barges. This would allow the terrorist an opportunity to deliver the chemical to the area of greatest importance or population concentration. Rail and truck delivery vehicles are particularly vulnerable since they don’t have any security after leaving the staging areas.
Two other types of chemicals also are transported across country and stored in bulk. Although these are not chemical weapons, the destructive potential they represent is massive.
Oxidizers:
- Oxygen
- Butadiene
- Peroxides
These easily can be combined with incendiary agents to produce "fuel-air" explosives capable of massive destruction. The secondary products of these explosions may liberate more chemical toxins.
Incendiary agents:
- Acetone
- Alkenes
- Amines
- Alkyl halides
- LPG, propane, and isobutane
- Gasoline and jet fuel
Food and Water Contamination
Certain chemical agents also can be delivered covertly through contaminated food or water. The military does not consider ingestion as a suitable route to employ chemical weapons because the effects are too slow and unpredictable. The terrorist does not have the same mission profile. Ingestion is an attractive route of dissemination, and water or food supplies or both can be contaminated. Cyanides, heavy metals, and aromatic hydrocarbons such as benzene are quite suitable for this contamination.
Contamination of water supplies often is deemed unsuitable for a potential terrorism attack because vast quantities of contaminating substances would be required to threaten a city’s water supply. Where current decontamination and water purification techniques make contamination with biological substances unattractive as terrorism tools, the same could not be said of tank car loads of toxic pollutants in municipal water supplies.
One such poison proposed for military use is fluoroacetic acid, which inhibits the tricarboxylic acid cycle. Symptoms of poisoning may include muscle twitching, visual disturbance, motor restlessness, seizures, fecal and urinary incontinence, coma, cardiac dysrrhythmias, cardiogenic shock, and finally death through respiratory or cardiac depression. The latency period is 30 minutes to 6 hours after ingestion.47 This poison is readily available in the Southwest as 1080 and is used to poison coyotes.
In 1999, the vulnerability of the food supply was illustrated in Belgium, where chickens accidentally were given animal feed containing dioxin-contaminated fat.48 This chemical contamination was not discovered for months. As a result, dioxin, a cancer-causing chemical that does not cause immediate symptoms in humans, likely was present in chicken meat and eggs sold in multiple parts of Europe during early 1999. This dioxin episode demonstrates how a covert act of food-borne chemical terrorism could affect commerce and human or animal health.
CDC Threat List
The CDC has prepared a list of chemical and biologic agents that might be used by terrorists, which often is used as a reference for training about how to manage these agents.49 The chemical agents chosen by the CDC range from warfare agents to toxic chemicals commonly used in industry. (See Table 7.)

Criteria for determining priority for inclusion in the CDC’s list of chemical agents includes:
- Chemical agents already known to be used as weaponry;
- Availability of chemical agents to potential terrorists;
- Chemical agents likely to cause major morbidity or mortality;
- Potential of agents for causing public panic and social disruption; and
- Agents that require special action for public health preparedness.
Personal Protective Gear
The use of personal protective equipment (PPE) to protect the airways, skin, and eyes is an indispensable component for the emergency department that receives chemically contaminated casualties. Ideally, each and every casualty that presents to the emergency department will be decontaminated completely prior to entry onto hospital property. For multiple reasons, including self-referral; bystander transport; transport by ill-equipped or ill-trained emergency service providers, fire, or police; and contaminated patients triaged as critical due to injuries and rushed to medical care, this prehospital decontamination likely won’t happen. The medical provider must assume that decontamination has not occurred and, hence, personal protection is needed.
The EPA has outlined detailed combinations of respirators and chemical protective attire that may be used in certain hazardous environments. These grades of protection are classified as levels A, B, C, and D. All emergency personnel who enter the contaminated zone or who have direct contact with contaminated patients will require PPE. Rescue workers required to enter a contaminated area require a greater level of protection than medical personnel providing care to contaminated or potentially contaminated patients.
The medical provider should realize that there is no perfect protection from a chemical agent. No one fabric or material protects against all chemicals. Each garment and protective device has made some compromises to offer protection against common chemical threats. The manufacturer of chemical protective garb assigns a lifespan in use to the garment to ensure that the protection remains intact. If the garment has exceeded the storage time or the exposure time, there is an increasing chance that the garment will fail. An outside chance also exists that terrorists have found a combination of chemicals that will penetrate the protective clothing used.
Chemical Protective Attire
Level A equipment provides the greatest degree of protection and consists of an encapsulated, vapor-impermeable, and chemical-resistant garment; double-layer, chemical-resistant gloves and boots; and a positive-pressure, SCBA. Airtight seals should be in place between the suit and the inner layer of hand, face, and foot protection. Level A protection usually is required only of those working in areas of very high concentrations of toxic agents. Level A suits and equipment will protect against most military and industrial compounds. Many municipal and corporate hazardous materials response teams in the United States have some level A capabilities.
Level B consists of a positive-pressure, self-contained breathing apparatus and a chemical-resistant suit with resistant gloves and boots. No airtight seals on the face, hands, and feet are necessary. Level B is used when full respiratory protection is required but skin exposure presents less danger. Many hazardous materials response teams outfit their decontamination squads with Level B protection.
Level C is required when concentrations of the toxic agent are expected to be much lower and there little likelihood of skin exposure. This is the usual situation for medical decontamination outside the emergency department. Level C protection consists of a full-face air purification device such as a canister mask and a nonencapsulating chemical-resistant suit with chemical-resistant gloves and boots. For medical decontamination of chemically contaminated patients, inexpensive, disposable, chemical-resistant, multi-layer polymer suits are available that correspond to level C protection.
Level D consists of standard work clothes and no respiratory protection. The level D usually consists of a long-sleeved shirt and long pants, so scrubs do not qualify as even level D protection. Level D is used only when there is no danger of chemical exposure.
Tyvek suits are completely inappropriate for work with chemical warfare agents and should be considered as Level D protection only. Tyvek provides no chemical protection because most chemicals can penetrate this material rapidly.
Respiratory Protection
Self-Contained Breathing Apparatus and Supplied Air Respirators. Supplied air respirators provide an uncontaminated source of air through an external supply. That air supply can be provided from a compressed air tank (SCBA) or by a hose from a pump located outside the contaminated zone.
Two types of masks are available for the supplied air respirator: a valve actuated air supply and a continuous flowing air supply. A valve actuated air supply is much more efficient air supply than the continuous-flow type. The fit of either type of mask is much less critical than with a cartridge respirator, since air is supplied to the user under pressure and leakage will be away from the airway.
The compressed air reservoir limits the duration of the use of the suit, since all air supply must be carried on the user’s back. Hoses provide much longer duration but may become tangled, break, rupture, and may be too short for effective separation of the air supply from the hazards. They may be useful for decontamination work where motion and exposure are decreased.
Cartridge Respirators. Cartridge respirators are the typical military style gas masks seen in the media. Cartridge respirators are inexpensive, portable, and easy to use and store. A cartridge respirator functions by filtering the air through a special sorbent material that binds the chemical vapors. The cartridge may be general purpose or may be chosen for the specific agent that is identified. Different cartridges must be used to protect from nerve gases, organic vapors, acid gases, chlorine, ammonia, and methylamine. The general-purpose cartridges often will have a markedly shorter effective protection time than the specific agent-related cartridges.
Cartridge respirators have a finite lifetime of use. If the concentration of the agent is high or if the cartridge is used for a long time, the chemicals will saturate the sorbent material. When this happens, the respirator starts to deliver contaminated air to the user. This limits cartridge respirators to short-term use and/or to low concentrations of chemicals in the air.
Cartridge respirators must be fitted to the user with an airtight seal, since the filter works with negative air pressure. Facial hair will destroy this seal and render the mask useless. A moderate amount of work is involved when inhaling across the pressure resistance of the cartridge.
Although masks may be found that only cover the nose and the mouth, the user still must have eye protection with goggles. There is little sense to using a half-face mask with the decreased protection provided to the user.
Gloves. Glove material is an important consideration, because the practitioner’s hands have the most contact with the patient. Unfortunately, no glove material can provide adequate protection against all chemicals. Many authorities recommend multiple gloves of multiple materials, but this is bulky and decreases the practitioner’s ability to feel a pulse or use tools. Nitrile gloves have much better chemical resistance than latex gloves. Thin, flexible nitrile gloves permit manual dexterity and offer significant resistance for chemical contamination. Latex gloves pass chemical agents so quickly that they are effectively useless.
Mission Oriented Protective Posture. The personal protective equipment used by the military is called mission-oriented protective posture (MOPP), which includes four levels. MOPP consists of charcoal-impregnated clothing, protective boots, protective gloves, hood, and a standardized military protective mask. This ensemble is designed to allow the soldier to continue a mission and protects against most chemical warfare agents if donned in time and if the soldier is not exposed to significant levels of agent for a protracted time. MOPP may not protect against some industrial chemicals.
Difficulties with Personal Protective Gear. Unfortunately, staff in full protective gear have difficulty performing tasks because of impaired vision and dexterity. Limitations of PPE are significant and include diminution of sensations such as hearing, vision, and touch; restriction of physical activity; fatigue; dehydration; heat-related illness; and adverse psychological effects, such as claustrophobia. An inappropriate feeling of immortality or false protection due to the protective wrappings can lead to costly or lethal errors.
References
1. McAdams AJ Jr, Joffe MH. A toxico-pathologic study of phosgene oxime. Army Chemical Center, MD; 1955. Medical Laboratories Research Report 381.
2. Okumura T, Takasu N, Ishimatsu S, et al. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med 1996;28:129-135.
3. The Terrorist Attack with Sarin in Tokyo, March 20, 1995. www.sos.se/sos/publ/refereng/9803020E.htm. Accessed 4-14-2003.
4. Harris LW, Heyl WC, Stitcher DL, et al. Effects of 1.1' -oxydimethylene bis-(4-tertbutylpyridinium chloride) (SAD-128) and decamethonium on reactivation of soman and sarin-inhibited cholinesterase by oximes. Biochem Pharmacol 1978;27:757-761.
5. Rickett DJ, Glenn JF, Houston WE. Medical defense against nerve agents: New directions. Milit Med 1987;152:35-41.
6. Rengstroff RH. Accidental exposure to sarin: Vision effects. Arch Toxicol 1985;56:201-203.
7. Duffy FH, Burchfiel PH, et al. Long term effects of an organophosphate upon the human electroencephalogram. Toxicol Appl Pharmacol 1979;47: 161-176.
8. McLeod CG. Pathology of nerve agents: Perspective on medical management. Fund Appl Toxicol 1985;5:S10-S16.
9. DeCandole CA, Douglas WW, Lovatt-Evans C, et al. The failure of respiration in death by an anticholinesterase poisoning. Br J Pharmacol Chemother 1953;8:466-475.
10. Ludomirsky A, Hlein HO, Sarelli P, et al. Q-T prolongation and polymorphous ("Torsade de Pointes") ventricular arrhythmias associated with organophorus insecticide poisoning. Am J Cardiol 1982;49:1654-1658.
11. Abou-Donia M. Organophosphorus ester-induced delayed neurotoxicity. Ann Rev Pharmacol Toxicol 1981;21:511-548.
12. Laskowski MB, Olson WH, Dettbarn WD. Ultrastructural changes at the motor end-plate produced by an irreversible cholinesterase inhibitor. Exp Neurol 1976;47:290-306.
13. Albuquerque EX, Akaike A Shaw K-P, et al. The interaction of anticholinesterase agents with the acetylcholine receptor-ionic channel complex. Fundam Appl Toxicil 1981;1:183-192.
14. Yang YC, Baker JA, Ward JR. Decontamination of chemical warfare agents. Chem Rev 1992;92:1729-1743.
15. Yang YC, Szfraniec LL, Beaudry WT, et al. Oxidative detoxification of phosphonothiolates. I Am Chem Soc 1990;112:662 1-6627.
16. Chemistry of VX. Mitretek Systems. www.mitretek.org/home.nsf/HomelandSecurity/VX. Accessed 4-8-2003.
17. Holstege CP, Kirk T, Sidell FR. Chemical warfare: Nerve agent poisoning. Crit Care Med 1997:13:923.
18. Weber LW, Zesch A, Rozman K. Decontamination of human skin exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in vitro. Arch Environ Health 1992;47:302.
19. Wester RC, Maibach HI. In vivo percutaneous absorption and decontamination of pesticides in humans. J Toxicol Environ Health 1985;16:25.
20. DuToit P, Muller F, Van Tonder W, et al. Experience with the intensive care management of organophosphate insecticide poisoning. S African Med J 1981;60:227-229.
21. Dunn MA. Pretreatment for nerve agents. Unpublished U.S. Army Information Paper 1987;AFZC-DMD.
22. Exner O, Benn MH, Willis F. Can J Chem 1968;46:1873.
23. Nerve Agents. FOA Briefing Book on Chemical Weapons. Organisation for the Prohibition of Chemical Weapons web site www.opcw.org/resp/html/nerve.html. Accessed 4-8-2003.
24. Sundwall A. Minimum concentrations of N-methylpyridium-2-aldoxime methane sulfonate (P2S) which reverse neuromuscular block. Biochem Pharmacol 1961;8:413-417.
25. Crook JW, Goodman AL, Colburn JL, et al. Adjunctive value of oral prophylaxis with oximes 2-PAM lactate and 2-PAM methanesulfonate to therapeutic administration of atropine in dogs poisoned by inhaled sarin vapor. J Pharmacol Ex Ther 1962;136:397-399.
26. Hoidal CR, Hall Kulig KW, et al. Pralidoxime chloride continuous infusions [letter]. Ann EmergMed 1987;16:831.
27. Kusic R, Jovanovic D, Randjeovic D, et al. HI-6 in man: Efficacy of oxime in poisoning by organophosphorus insecticides. Hum Exp Toxicol 1991;10: 113-118.
28. Nerve Agents. Source: FOA Briefing Book on Chemical Weapons. Found at Organisation for the Prohibition of Chemical Weapons web site www.opcw.org/resp/html/nerve.html. Accessed 4-8-2003.
29. Kaplan T. The Tylenol Crisis: How Effective Public Relations Saved Johnson & Johnson. Accessed at www.personal.psu.edu/users/w/x/wxk116/tylenol/crisis.html. Accessed 4-8-2003.
30. Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med 1991;325:1761-1766.
31. Bermudez RM, Cabrera CA. Treatment of burns. N Engl J Med 1997;336: 1392-1393.
32. Recommendations for protecting human health against potential adverse effects of long term exposure to low doses of chemical warfare agents. MMWR Morb Mortal Wkly Rep1988;37:72-74.
33. Fligner CL, Luthi R, Linkaityte-Weiss E, et al. Paper strip screening method for detection of cyanide in blood using CYANTESMO test paper. Am J Forensic Med Pathol 1992;13:81-84.
34. Hall AH, Rumack BH. Clinical toxicology of cyanide. Ann Emerg Med 1986;15:1067-1074.
35. Chen KK, Rose CL, Clowes GHA. Methylene blue, nitrites, and sodium thiosulfate against cyanide poisoning. Proc Exp Biol Med 1933;31:250-252.
36. Berlin CM Jr. The treatment of cyanide poisoning in children. Pediatrics 1970;46:793-796.
37. Cottrell JE, Casthely P, Brodie JD, et al. Prevention of nitroprusside-induced cyanide toxicity with hydroxycobalamin. N Engl J Med 1978;298:809-811.
38. Graham DL, Laman D, Theodore J, et al. Acute cyanide poisoning complicated by lactic acidosis and pulmonary edema. Arch Intern Med 1977;137: 1051-1055.
39. Mushett C. Antidotal efficacy of vitamin B12 a (hydroxocobalamin) in experimental cyanide poisoning. Proc Soc Exp Biol Med 1952;81:234-237.
40. Sauer SW. Hydroxocobalamin: Improved public health readiness for cyanide disasters. Ann Emerg Med 2001;37:635-641.
41. Jouglard J, Fagot G, Deguigne B, et al. L’intoxication cyanhydrique aigue et son traitement d’urgence. Marseille Medicale 1971;9:571-575.
42. Vogel SN, Sultan TR, Ten Eyck RP. Cyanide poisoning. Clin Toxicol 1981; 18:367-383.
43. Bismuth C, Cantineau J-P, Pontal P, et al. Priorite de l’oxygenation dans l’intoxication cyanhydrique. J Toxicol Med 1984;4:107-121.
44. Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med 1987;5:115-121.
45. Kindwall EP. Carbon monoxide and cyanide poisoning. In: Davis JC, Hunt TK, eds. Hyperbaric Oxygen Therapy. Bethesda MD: Undersea Medical Society; 1977:177-190.
46. Grimaldi JV, Gugliotta G. Chemical plants feared as targets. The Washington Post. December 16, 2001:A01.
47. Weger NP. Therapy of chemical warfare agent poisoning. Med Bull US Army, Europe. 1981;38:30-32.
48. Ashraf H. European dioxin-contaminated food crisis grows and grows [news]. Lancet 1999;353:2049.
49. Chemical Agents. Centers for Disease Control and Prevention. www.bt.cdc.gov/Agent/agentlistchem.asp. Accessed 4-8-2003.
