Bites and Stings: An Overview of Close Encounters with Nature. Part II
Authors: Gary D. Hals MD, PhD, Attending Physician, Department of Emergency Medicine, Palmetto Richland Memorial Hospital, Columbia, SC; and Eric Brittain, MD, Resident Physician, Department of Emergency Medicine, Palmetto Richland Memorial Hospital, Columbia, SC.
Peer Reviewers: J. Stephan Stapczynski, MD, Chair, Emergency Medicine Department, Maricopa Medical Center, Phoenix, AZ; and Steven M. Winograd, MD, FACEP, Attending Physician, Emergency Department, Adena Regional Medical Center, Chillicothe, OH.
Part I of this two-part series on bites and stings covered the diagnosis and management of mammalian bites and insect stings. Part II will examine the management of snake and reptile bites as well as bites and stings from marine animals. — The Editor
Reptiles
Non-venomous Snakes. By nature, non-venomous snakebites normally are not clinically significant. North American non-venomous snakes are constrictors (i.e., corn snakes, king snakes) or snakes that eat their prey live (i.e., garter snakes). Their teeth are very small and curved in toward their throats, and function to keep prey from escaping once captured. Bite wounds consist of several very small, needle-like puncture wounds, sometimes in parallel rows if both sides of the jaw connect with the patient. These patients most often present to the emergency department (ED) because they are not sure if the snake that bit them was venomous or not. Most of their care centers on identification of the snake (See section on Snake Identification), and once assured it was not venomous, reassurance that no harm will come to them. Prophylactic antibiotics are not indicated, and bite wounds require no special treatment. As with all other bites and stings, tetanus prophylaxis is recommended if not already up to date.
Large Constrictors. The only exception to the above discussion would be large constrictors, such as boas or pythons, that are not native to the United States. Although not native, there are a great many of these snakes in captivity as pets in the United States. Most major U.S. cities have reptile shows yearly where these animals can be purchased for as little as $10. Boa constrictors, ball pythons, and Burmese pythons are three of the most popular examples. While all three are similar in size as hatchlings, ball pythons only reach 4-5 feet in length and weigh a few pounds. In contrast, boas can grow to be 10 feet and 40-50 pounds, and Burmese pythons easily can reach 12-18 feet and more than 150 pounds as adults. Further, when properly fed, these animals can reach these lengths in as few as 2-3 years. Well-fed large snakes can be quite docile depending on the species. Boa constrictors and Burmese pythons are well known for their docile dispositions, while reticulated pythons tend to be more aggressive. Still, as with all animals, the behavior of individuals can vary. Some owners mistakenly believe that withholding food or keeping larger species in small cages will stunt their growth enough to keep them from getting large. While spacing out feedings will make these snakes grow more slowly, they also will be quite hungry all of the time, making them mean and unpredictable. Snakes treated this way are much more likely to strike at anything warm, as they might behave in the wild under stress. If uninformed or given bad advice, it is easy to unintentionally purchase an animal that quickly grows faster than one’s ability to care for it. While many snake owners are well informed and conscientious, some purchase these large snakes without adequate knowledge of how to care for them. Each year in the United States, several people are attacked by their pets, and deaths are not unusual. Most attacks occur through inappropriate feeding where snakes are hand-fed or handled while the scent of prey is still on a patient’s hands. These snakes hunt primarily by smell, and mistakenly may grab a hand when it smells like a mouse, rat, rabbit, chicken, or other prey. Although their mouths and teeth are designed the same as their North American counterparts, their large size makes their bites much more significant. The head of a large adult constrictor can be 6 inches across. Large lacerations can result, even injuring deep structures like tendons, nerves, and arteries. Unlike smaller snakes, bites from larger constrictors may need X-rays to evaluate for retained tooth fragments.1 Few studies exist, but they support that these bite wounds do not benefit from prophylactic antibiotics.2
Snake Identification. Of the nearly 200 snake species native to North America, only a handful are venomous (28 pit vipers and two coral snakes—See next section). Be aware that only Maine, Alaska, and Hawaii have no native pit viper species. If dealing with an exotic snake species, identification can be much harder. They only general rule here is to consult an expert, such as herpetologist at a local zoo or a toxicologist. There is now a national toll-free number (800-222-1222) that automatically connects to the nearest poison control center that may be helpful. The Arizona Poison and Drug Information Center (520-626-6016) also is a good back-up resource for venomous snakebite treatment, and maintains a database on antivenom availability in the United States.
Identification of North American snakes as venomous vs. non-venomous generally can be done using several anatomic features of venomous snakes; however, some caveats need to be made. Exceptions to these rules exist and will be listed. Identification often requires rather close examination of the snake, and one must be aware that this can be risky in itself. Do not handle the snake if not trained in snake identification. Dangerous envenomations (even deaths) have been reported from reflexive bites of severed snake heads. Further, several snake species are known to play dead convincingly and may come back to life unexpectedly. When in doubt, leave the snake alone and treat the patient based on symptoms. (See section on Treatment.) Likewise, one should discourage retrieval of a snake to the ED for identification. The incorrect snake may be brought back, leading instead to false assumptions and improper treatment. This also is can produce additional snakebite victims for treatment in the ED.
The most obvious identifier of a venomous snake is the presence of rattles on its tail. No snakes other than rattlesnakes have this feature, but be aware that rattles may be missing entirely from previous trauma. Also, a hatchling rattlesnake only possesses a button on the end of the tail and does not develop its first rattle segment until its first shed (about 1-2 weeks of age). To make things even more complicated, one subspecies (Santa Catalina rattlesnake) actually has no rattles naturally (they possess the tail bud at birth, but subsequent rattle segments develop but do not attach to the tail). While rattles accurately identify rattlesnakes, cottonmouths (or water moccasins), copperheads, and coral snakes all are venomous but have no rattles. Further, most all North American non-venomous snakes, as well as copperheads and cottonmouths, tend to rattle their tails when disturbed. If done around dry leaves, they can produce a rattling sound that initially may be confused with a true rattlesnake. Obviously, this benefits the snake if it is misidentified by a predator as a more deadly animal. Other methods of identification include the shape of the eye, pattern of belly scales on the tail, and presence of heat-sensing pits on the face. (See Figures 1-3.)
Figure 1. Features of Most Venomous U.S. Snakes
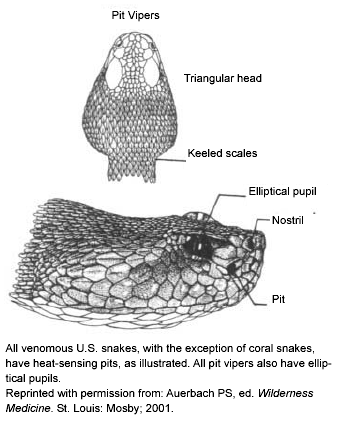
Figure 2. Illustration of Range
of Heat-Sensing Pits
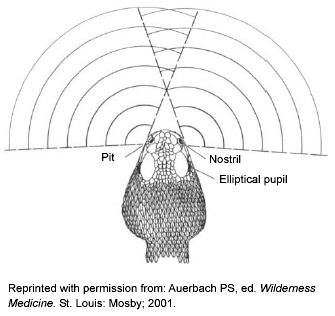
Figure 3. Differences Between Venomous
and Non-venomous Snakes
Three of the four groups of venomous snakes native to North America (i.e., rattlesnakes, cottonmouths, and copperheads) are pit vipers. The only exceptions are the three brightly colored coral snake species. (See section on Venomous Snakes.) Pit vipers are named for the heat-sensing pit located between the eye and the nostril on each side of the head. (See Figure 1.) The pit allows the snake to "see" in infrared and locate warm-blooded prey more efficiently in the dark. All pit vipers have pits 100% of the time, but if the head is damaged they may not be visible. Pits look like enlarged nostrils, and so require a relatively close look to be identified. Besides coral snakes, be aware that non-native vipers (vipers from Europe, Asia, and Africa) have no pits.
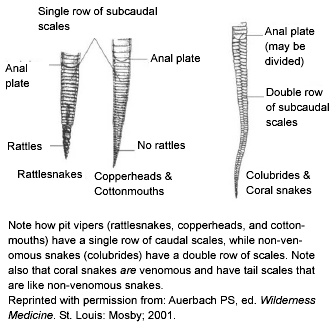
North American pit vipers also have elliptically shaped pupils similar to a cat’s eyes, whereas non-venomous snakes have round pupils. Again, coral snakes are the exception, as they have round pupils. Assessing pupil shape also requires close proximity to the snake’s head, which is not advisable with a live snake. Similarly, the numbering of the belly scales on the tail accurately can identify a venomous pit viper. Non-venomous snakes have a double row of scales below the anal plate, while venomous snakes have a single row. (See Figure 3.) Once again, the coral snake remains the exception as a venomous snake with a double row of belly scales on the tail. And once again, belly scale patterns are a useful tool that requires closer contact than is recommended with a live snake.
Lastly, a warning that some methods of identification can be misleading. Head shape can be used to identify venomous snakes, as all pit vipers have triangular-shaped heads while non-venomous snakes have a streamlined shape. (See Figure 1.) This is not recommended as an identification tool for the novice as many live non-venomous snakes flatten their heads when threatened and effectively make themselves look like venomous snakes. In addition, coral snakes are again the exception as their head shape is identical to non-venomous snakes. Likewise, coloring or the pattern on the snake can identify the species of the snake, but the novice should not use this as definitive identification alone. Color patterns change greatly with age of the snake, time of the year, and geographical location. A young snake typically looks quite different than it will as an adult, and considerable overlap in appearance of venomous and non-venomous snakes at various stages of their lives can exist. The same species from different states also may look at first like a different snake. In addition, many mimics of the coral snake exist that can be difficult to distinguish at first glance, thus coloring alone is not reliable. All venomous snakes have fangs, but while fangs can be large (1-1 ½ inches) in pit vipers, they retract into fleshy coverings, and the snake voluntarily can keep one or both retracted when opening its mouth. Thus, absence of notable fangs when the snake opens its mouth may not be a sign of a non-venomous animal. Further, coral snakes have relatively small fangs that may not be seen easily even with close inspection. Snakes also naturally lose fangs over their lifetime, and even absence of fangs in a dead specimen may be only a sign of recent trauma.
Venomous Snakes. U.S. residents are relatively lucky compared to a large portion of the world when it comes to venomous snakes. The 2002 American Association of Poison Control Centers (AAPCC) report showed only two deaths for the 2325 venomous snakebites reported in the United States.3 The AAPCC numbers are an underestimate of the true number of venomous snakebites in the United States, estimated at 8000 per year.4 Still, this compares to an estimated 2.5 million venomous snakebites and 125,000 deaths worldwide,5 and actual numbers may be even larger. The majority of these deaths occur from lack of treatment resources, and the fact that many of these non-North American snakes tend to be more deadly than North American ones. Risk factors for venomous snakebite in the United States include male sex, younger age (17-27 years), and alcohol use as a significant co-factor.6 More than 95% of bites occur on the extremities, with bites peaking in months of July and August. All states but Alaska, Maine, and Hawaii have at least one species of native venomous snake.
Native U.S. venomous snakes can be divided into two groups: pit vipers (Crotalidae) and coral snakes (Elapidae family). The pit vipers are further divided into rattlesnakes, cottonmouths (or water moccasins), and copperheads. Each group will be discussed individually, and then an overview of general treatment recommendations will be given. In general, pit vipers can be approached as a group, with a few exceptions that will be discussed, but coral snakes are treated much differently than any other venomous U.S. snake. (See section on Coral Snakes.) Remember that all exotic (non-native) venomous snakebites will need to be approached differently than native U.S. venomous snakebites. (See section on Exotic Venomous Snakes.) The pit viper antivenom, CroFab, is designed for native U.S. species, and any other antivenom not specifically produced for the snake species in question will be ineffective. In other words, viper antivenom will not treat a cobra bite, etc. (See section on Antivenom.)
Rattlesnakes (Crotalus and Sistrurus). There are roughly 15 species of rattlesnakes native to the United States. Contrary to most people’s perception, these snakes are rather reclusive and do not strike unless provoked or threatened. The rattle presumably evolved as a warning to keep large herbivores from accidentally trampling them. The snake uses its venom to kill and begin digestion of its prey (small rodents), and would rather not envenomate something it cannot eat if given the choice. Thus the rattle can warn other animals away, allowing the snake to save the venom for prey. Most people are surprised to learn the snake also has voluntary control over whether venom is injected and the amount injected in each bite. (See section on Dry Bites.)
Rattlesnakes are native to every state except Maine, Alaska, and Hawaii. Examples of more well-known U.S. rattlesnakes include the Eastern and Western diamondback, the sidewinder, the timber rattlesnake (or canebrake), and the Mojave rattlesnake. They vary in size from the pygmy rattlesnake, 15-20 inches as adults, to the diamondback, which averages around 6 feet but has been recorded at 8 feet long. The rattle is composed of loosely overlapping keratin plates. While it is true that a new segment is added each time the snake sheds, this may occur more than once per year, and segments can be broken off, making segment counting an unreliable method of estimating a snake’s age. It also is incorrect that the rattlesnake will always rattle before striking in defense. Rattlesnakes hibernate from around November to April in large underground cavities and are mostly nocturnal. They have rather large front fangs that are hinged and covered in tissue sheaths. Their strike is extremely fast, measured at 8 feet per second,7 but they can only crawl about 3 miles per hour—much slower than the normal person walks.
The venom of rattlesnakes is designed to kill and begin digestion of their prey. To say it is complex is an understatement. It is composed of many proteins, peptides, amino acids, and metals such as zinc, copper, and magnesium. These compounds act in unison to produce three general categories of envenomation results: cytotoxic, hemorrhagic, and neurotoxic effects. Cytotoxins damage endothelial cells and produce marked local swelling and lysis of red blood cells, platelets, and mitochondria. Proteolytic enzymes damage connective tissue, allowing the venom to spread. They also destroy muscle and subcutaneous tissue, producing necrosis. Hemorrhagic compounds induce extravasation of blood and ecchymosis at the bite site. Thrombin-like effects also are produced directly by crotalid venom and result in widespread intravascular coagulation (DIC). Thrombocytopenia also can follow envenomation and can be used as a marker for severity. The more rapid and severe the fall in platelets, the more dangerous the envenomation. Neurotoxicity is produced by blockage of presynaptic nerve transmission. However, the exact composition of the venom, and therefore its effects, vary much more than one might expect with the species, age of snake, diet, season, and even geographic location. The Mojave rattlesnake gives the best example of how much variability can exist.
While somewhat similar in coloring and pattern to a small Western diamondback, the Mojave rattlesnake has the distinction of possessing the most potent neurotoxic venom of all rattlesnakes. It is estimated to be 10 times more potent than any other North American venomous snake on a mg/kg basis.8 Mojave rattlesnakes actually are divided into type A and type B snakes even though they are the same species. The difference is based on the geographic location: Type A snakes live primarily in southern California, Nevada, Utah, Arizona, Texas, and New Mexico, while type B snakes live only in south-central Arizona. To make matters more complicated, individual snakes with features of both type A and type B snakes also have been found. Type B snakes produce venom that acts similar to other rattlesnakes (hemolytic and proteolytic effects), but type A snakes produce a primarily neurotoxic venom with less effect on local tissues. The neurotoxin in type A snakes is termed Mojave toxin and acts presynaptically to inhibit neuromuscular transmission, causing muscle paralysis. Thus bites by a type A snake will have much fewer local effects and initially may give false reassurance that no venom was injected in the bite. (See section on Dry Bites.) Further, like coral snakes, symptoms of envenomation may be delayed hours after the bite.
Clinical Effects. There are a wide variety of signs and symptoms of pit viper envenomation. The effect in any one case will depend on a number of factors: the species of the snake, its size, health, and age, as well as the location of the bite on the patient and the patient’s underlying health. The location of the bite also can give clues as to the cause; most accidental bites occur on the lower extremities while most bites to the upper extremity occur during intentional interaction with the snake.9 The most common profile in the United States is a young intoxicated male bitten on the hand while trying to interact in some (inappropriate) way with the snake. Bites to the head and neck are less common, but often rapidly will produce severe symptoms. Bites that inadvertently inject venom directly into a vein or artery may produce systemic symptoms in only a few minutes; these patients may not survive long enough to reach the ED.
Overall, most pit viper bites produce symptoms in 30-60 minutes. Only a brief overview of symptoms is presented, and readers interested in detailed descriptions are referred to detailed text.7 Initial findings include burning pain and swelling at the bite site, but in the case of a Mojave rattlesnake bite type A, snakebites may produce little or no pain. For all rattlesnakes, ecchymosis and bullae may take several hours to develop. Beware that in some cases, the edema itself may not appear for several hours and that seemingly mild envenomations suddenly may take a turn for the worse. Mild systemic effects include nausea, vomiting, paresthesias (tingling of the mouth, scalp, or feet), fasciculations, and weakness. Bites from larger species may produce a rubbery, minty, or metallic taste. More severe systemic effects include altered mental status, tachycardia, tachypnea and respiratory distress, and hypotension. Laboratory abnormalities include coagulopathy (prolonged prothrombin time [PT] or partial thromboplastin time [PTT] and decreased platelets), elevated white blood cell (WBC), creatinine kinase (CK) levels, and transaminases. The patient who has underlying medical problems also may experience complications of these conditions, i.e., acute myocardial infarction (MI), acute renal failure, asthma attacks, etc. Symptoms are used to grade the level of envenomation, which is key for defining treatment. (See section on Grading of Envenomation and Table 1.)
Cottonmouth (Water Moccasin) (Agkistrodon piscivorus). The cottonmouth or water moccasin is named for its habit of coiling up and exposing the white interior of its mouth when threatened. They are found in the southeastern United States as far west as Texas and as far north as southern Missouri. They are darkly colored (brown or olive) with a yellowish belly as adults. Hatchlings can be much brighter with bands of alternating color looking similar to their cousin the copperhead, but are still darker than copperheads. Like young copperheads, they also have a bright yellow or lime-green tip of the tail used to lure prey. Adults are typically around 3 feet, but have been recorded at 6 feet. They have a heavy body like larger rattlesnakes, and have a reputation for being aggressive. This means they tend to stand their ground when approached. They often are found in or around water. The AAPCC report shows 173 cottonmouth bites treated in 2002—much fewer than both copperheads or rattlesnakes. Venom is similar to rattlesnake venom, but less toxic, and no deaths from cottonmouths have been documented since the first AAPCC report in 1983. Symptoms and treatment of envenomation are the same as for the copperhead. (See next section.)
Copperhead (Agkistrodon contortri). Copperhead snakes are a close relative of the cottonmouth, and both are considered moccasin-type animals. They are found in the southeastern United States and range as far west as Texas and as far north as New York. They are named for the copper colored bands that alternate with darker brown bands along the length of the body. As hatchlings, they also have a bright yellow tip to the tail, which they use as a lure to bring prey (lizards) into striking range. They are not as big as full-grown rattlesnakes (at fewer than 3 feet as adults) and tend not to be as heavy as cottonmouths. The AAPCC 2002 report shows 889 patients were treated for copperhead bites, compared to 1150 for rattlesnake bites and 173 for cottonmouth bites. While venom is similar to other pit vipers, copperhead bites tend to be less potent, and no deaths have ever been reported to the AAPCC.
Symptoms of envenomation include pain and swelling at the site, which can progress rapidly. Cytotoxins in the venom produce ecchymosis and hemorrhagic bullae similar to rattlesnakes. Systemic signs of tachycardia and hypotension can occur. Laboratory studies are the same as for rattlesnake envenomation. (See section on Grading of Pit Viper Envenomation.) One study of cottonmouth and copperhead bites demonstrated the lack of systemic toxicity in most bites.10 A series of 55 patients envenomated by these snakes found that 95% of bite victims had local swelling and pain, but only 14% had any systemic symptoms (nausea, vomiting, or tachycardia). Further, none of the 55 had any laboratory abnormalities, and none developed any significant tissue damage or loss of function. Although symptoms are generally less severe than with rattlesnake bites and most bites do not meet criteria for antivenom use, young or elderly patients may have more severe symptoms. (See section on Antivenom.)
Coral Snakes (Micrurus). Coral snakes are a member of the Elapidae family and are related to cobras, kraits, and mambas. Coral snakes are notable exceptions to pit vipers in many ways. They are brightly colored, small, nocturnal snakes with neurotoxic venom that is more like cobra venom than pit viper venom. They also have no heat-sensing pits. Only three species of coral snake are found in the United States. The Eastern coral snake (Micrurus fulvius fulvius) is native to North Carolina, South Carolina, Florida, Louisiana, Mississippi, Georgia, and Texas. The Texas coral snake (Micrurus fulvius tenere) is native to Texas, Arkansas, and Louisiana. The Western or Sonoran (Micruroides euryxanthus) coral snake is native only to Arizona and New Mexico. It is important to note that only bites of the Eastern or Texas coral snakes are considered dangerous and are treated with antivenom. Bites of the Western or Sonoran coral snake are considered mild, and no fatalities have been documented. All coral snakes are small and thin, only 2 feet long when adults, and resemble harmless king snakes in appearance. Color bands alternate in a red, yellow, black, yellow, red repeating pattern, completely encircling the body, and the snout is always black. Several mimic milk snakes and king snakes (scarlet king snake, Mexican milk snake), and an easy phrase to tell them apart is, "Red touch yellow, kill a fellow; red touch black, venom lack." Native U.S. mimic snakes also always have red snouts, whereas true coral snakes have black snouts. Notable exceptions are the Mexican coral snake, which lacks much yellow coloring and whose red bands lie next to black ones, and the Mexican milk snake (non-venomous), which has a black nose.
Fangs are small (1-3 mm) and fixed in position, unlike large retractable pit viper fangs. They may be difficult to see, even in dead specimens. Unlike other snakes, the coral snake usually must hold on and chew for effective envenomation to occur. One study found that 85% of victims of a coral snake bite reported that the snake hung on and actually had to be actively removed.11 Even so, bite wounds are very small and may be overlooked in the ED. When bites do occur, they tend to be in warmer months (April-October) and occur after dark. Coral snake bites are rare, accounting for fewer than 1% of all venomous snakebites in the United States.12 The 2002 AAPCC data list 88 coral snake bites that year.3 The relatively low number of bites is due in part to their nocturnal, burrowing lifestyle, and the fact that they are small, shy by nature, and rarely encountered by people. This is fortunate, as the neurotoxic venom they inject often produces very few local symptoms. However the Eastern coral snake venom is very potent and it is estimated from mouse studies that an average adult Eastern coral snake carries enough venom to kill 4-5 adults.13 Of all the native venomous snakes, only the Mojave rattlesnake produces more potent venom. The Texas coral snake’s venom is similar to the Eastern coral snake, but is considered less potent. While venom from the Sonoran coral snake also is similar, it only seems to produce very mild if any CNS effects. Thus Sonoran coral snake bites are considered less dangerous. As discussed below, the Eastern and Texas coral snakes are the only native snakes whose antivenom is given for true bites regardless of patient signs and symptoms. Symptoms are well documented to be delayed for 12 hours, and then rapidly to progress.14 Even worse, when symptoms develop they often are resistant to antivenom treatment.
Do not confuse the envenomation grading scale (see Table 1) developed for evaluation of pit viper bites for evaluation of a coral snake bite; it is not applicable, and likely will result in inappropriate treatment. In nearly half of true envenomations, there is no redness or edema at the bite site because coral snake venom contains very few cytotoxins. Bullae are seen in only 5% of cases.11 Ptosis or nausea/vomiting often are the earliest signs of envenomation. Signs of coral snake envenomation also include sweating, headache, or abdominal pain. Parathesias or altered mental status can develop (decreased sensorium or euphoria followed by focal neurologic deficits). Cranial nerve dysfunction (i.e., ptosis, dysphonia, dysphagia) or peripheral motor nerve deficits often are seen. Proximal muscle paralysis can occur with subsequent respiratory failure, aspiration, and death. Respiratory failure ultimately is the most common cause of death,15 but no deaths have been reported after introduction of coral snake antivenom. Further, supportive treatment alone can be effective as most deaths are due to failure to initiate ventilatory support early on when symptoms develop. While paralysis may last only 3-5 days, complete neurologic recovery from effects of the venom may take weeks. In contrast to pit viper venom, coral snake venom contains few if any hemotoxins, and coagulopathies are not seen in coral snake envenomation. Thus, the only useful laboratory test to obtain when treating a patient bitten by a coral snake would be an arterial blood gas (ABG) to assess respiratory status.
Exotic (Non-native) Venomous Snakes. Herpetology as a hobby has grown significantly in the past two decades. Most large U.S. cities now have reptile shows where virtually any adult can purchase a wide variety of non-native (exotic) snakes and other reptiles. As a result, the number of patients seen for bites from exotic snakes in EDs across the country has grown. Data show that before 1960, only 4% of bites were from non-native species.16 By 1972, 15% of 410 patients hospitalized for snakebites were bitten by non-native species.17 In 1984 it was estimated that 30 patients were treated in the United States for bites from exotic snakes,18 but data from the 2002 AAPCC Toxic Exposure Surveillance System found 125 such cases.3
Another way to think of this issue is to consider the number of snakebites treated from captive animals, since non-native species are held in captivity. One author reviewed consultations for non-native venomous snakebites from 1977 to 1995 and found that these species were responsible for 33% of the consultations.19 Further, fully 48% of all bites (native and non-native) were from captive species. The current edition of Auerbach’s Wilderness Medicine put this issue in perspective by stating: "An ED physician in an urban hospital in the eastern or midwestern United States is almost as likely to be confronted with a bite of an exotic venomous snake as with that of a species native to North America."16
The most popular venomous non-native snakes sold in the United States are cobras and vipers (puff adder, gaboon viper, and rhinoceros viper) from Africa and Asia. There are some data to show that cobra bites are very common among non-native bites, with cobras causing 40% of non-native snakebites in one series.19 Interestingly, native U.S. venomous snakes are very popular in Europe, with one report on snakebites in France showing that Crotalids (U.S. rattlesnakes, copperheads, and cottonmouths) were responsible for a large number of non-native snakebites treated there.20
Now the question arises, what does one do when confronted with a victim bitten by a non-native species? The key is identification of the species, as treatment is based strictly on the snake involved. This is very important; antivenom for exotic snakes is species- and even region-specific. (See section on Exotic Snake Treatment.) In other words, antivenom for a Pakistani cobra is unlikely to be effective if used for a Chinese cobra bite. The best approach is to ask the patient what type of snake caused the bite. In most cases, the patient can provide reliable information on the species of snake he or she owns. In short, if the patient says he or she was bitten by an Indian spectacled cobra or West African gaboon viper—believe him or her. General treatment measures for exotic venomous snakebites are given in the next section.
Treatment of Venomous Snakebites. Dry Bites. Many people are surprised to find that in about 25% of pit viper bites and nearly 50% of coral snakebites absolutely no venom is injected.4 This is not a feature limited to U.S. snakes; the same occurs for venomous snakes throughout the world.21 These bites are termed dry bites, and cause no problems other than fang marks. Dry bites can occur because the snake’s venom glands are surrounded by voluntary muscles that control the amount of venom injected in each bite. The snake actually varies the amount of venom injected in relation to the size of the prey and the purpose of the bite: for feeding or for defense. Research has shown that rattlesnakes inject significantly more venom into larger mice than into smaller mice.7 It is possible that a person even may have multiple fang marks from several bites without envenomation. Thus, an asymptomatic patient presenting with the history of a venomous snakebite may require a period of observation (at least 8 hours) to see if envenomation has occurred or not. Two notable exceptions are young children or patients bitten by a Mojave rattlesnake, where both should be observed for 24 hours, (See Table 2.)
Table 2. Disposition of Patients Treated
for Venomous Snakebite

Prehospital Treatment. A wide variety of treatments for snakebite victims in the field have been proposed and tried throughout the years. These include incision of the fang marks and sucking out venom (or some variation of extraction devices), tourniquets, constriction bands, electrotherapy, cryotherapy, and so on. Electrotherapy and cryotherapy clearly are not beneficial. The literature on the other techniques can be contradictory, but most recent articles suggest that there is no clear benefit.4,6 An exception to this is the Australian compression technique, which has been shown to be helpful with envenomation by snakes with primarily neurotoxic venom (most cobras, mambas, kraits, sea snakes, and Australian snakes such as the taipan and tiger snake). It consists of placing an elastic wrap bandage (or similar bandage) around the bitten extremity immediately after the bite. The wrap is applied only as tightly as for an acute sprain, begun at the bite site, and wrapped outward to include the hand or foot and the proximal joint (i.e., elbow, knee, shoulder, or hip). The extremity also is splinted, and spread of the venom is slowed by this technique. Be aware that this technique is not well-studied with venoms that produce local damage, with some studies suggesting local tissue loss is magnified by keeping a higher concentration of the venom at the bite site.20
Grading of Pit Viper Envenomation. As pit vipers account for 98% of all bites treated in the United States,22 an envenomation grading system has been developed for use with North American pit viper bites. It is important to remember not to use this grading system for bites from any other venomous snakes, especially coral snakes, as it likely will lead to inappropriate treatment. Also, type A Mojave rattlesnakes (see section on Rattlesnakes) have a significant neurotoxic component to their venom, and this grading system may not apply well in their case either. The grading system is designed to assist with decisions on antivenom use and gives a rough idea of how much antivenom may be required. (See Table 1.) In general, there are four grades of bite symptoms from no symptoms (dry bite), to mild, moderate, and severe symptoms. Dry bites are described in the previous section. Mild envenomations have only local pain and swelling with no systemic or laboratory abnormalities. Moderate envenomations have progressive swelling at the site, mild laboratory changes, and non-life-threatening systemic symptoms. It is good practice to begin marking any swelling around the bite site with a marker to keep track of progression. Times also can be written to provide other physicians with objective measures of the swelling. Severe envenomations have rapid local swelling, dramatic laboratory changes, and dangerous systemic symptoms. (See Table 1). Each patient with an envenomation is different, and it is critical to remember when using this system that the grading ultimately is determined by the most severe sign, symptom, or laboratory finding in each category. In other words, if one patient only has mild local swelling and normal laboratory test results, but has hypotension (systolic BP less than 80 mmHg), then the patient is classed as a severe envenomation. Also be aware that not all severe envenomations may appear so in the first 1-3 hours. Patients with only mild symptoms initially need close monitoring so that if rapid progression occurs, they will be treated as quickly as possible.
One often sees significant swelling of a limb after envenomation that can mimic compartment syndrome, but compartment syndrome after snakebite actually is not a common occurrence. One must be vigilant, though, as typical signs of compartment syndrome (i.e., pain, paresthesias, pallor, cool skin) also may be present to some degree as a result of the action of the venom. Compartment pressures should be measured to ensure that the diagnosis of compartment syndrome is neither overlooked nor over-diagnosed. Increased compartment pressures (30-40 mmHg) can be treated with additional antivenom dosing, elevation of the limb, and 1-2 g/kg of mannitol (if the patient is hemodynamically stable).7 If pressures are not reduced below 30 mmHg by these maneuvers, the patient should be referred for further evaluation by an orthopedist or general surgeon.
Laboratory tests are ordered to evaluate the severity of envenomation in most cases. The exception is for coral snake bites, where laboratory abnormalities are not a feature of envenomation. A typical panel for pit viper envenomation would include: complete blood count (CBC), PT, PTT, fibrinogen and fibrin degradation products (PDP), d-dimer, electrolytes, blood urea nitrogen (BUN), creatinine, CK, urinalysis (UA) for myogloinuria, with an electrocardiogram (ECG) and ABG only in more severe cases. Chest X-rays may be obtained in patients with respiratory symptoms, and plain films of the bite may be useful to rule out retained fangs.
Antivenom. There are currently three types of antivenom available for use with envenomation by North American snakes: coral snake antivenom (see next section), and two types of pit viper antivenom. The older type of antivenom is a polyvalent antivenom (Antivenin Crotalide Polyvalent) manufacturered by Wyeth. Rumors exist that the manufacturer recently has stopped production of this antivenom, but this is not the case. Emergency supplies are maintained by the manufacturer, but may take 24 hours for delivery. The newer type of antivenom is CroFab (Crotalidae Polyvalent Immune Fab Ovine). Any patient to whom antivenom is given should be referred for admission, usually to an intensive care unit (ICU) setting. A final common point for both types of antivenom: The dosing is the same for pediatric and adult patients. The amount of antivenom required is a function of the dose of venom received, not a function of the patient’s size.
Polyvalent Antivenom. The polyvalent antivenom is made using the same method since production began in 1954.23 Venom from four species (Eastern and Western diamondbacks, South American rattlesnake, and the fer-de-lance) is injected into horses, and antibodies are purified to make the antivenom. Both the South American rattlesnake and the fer-de-lance are not native U.S. snakes, but do account for numerous snakebites in South America. Even so, through cross-reactivity the polyvalent antivenom is effective against all envenomations from any viper native to North, Central, or South America. (However, it is important to note that the polyvalent antivenom does not provide as effective coverage for type A Mojave rattlesnake envenomations as does CroFab.) Due to imperfections in the purification process, only 15-25% of the resulting antivenom contained IgG.24 Other proteins contained in the polyvalent antivenom are responsible for most of the anaphlyactoid and serum sickness reactions.
The manufacturer recommends skin testing for individuals in whom antivenom is to be used, but this is not always endorsed by practitioners. Advocates suggest that skin testing will help identify patients who have an obviously severe hypersensitivity so that caregivers can be ready to treat allergic reactions. Opponents of skin testing point out that skin testing is an inaccurate way to predict possible allergic reactions, and antivenom will be given anyway for serious envenomations.23 Up to 28% of patients will have a negative skin test and still have an acute allergic reaction, and up to 30% who have a positive skin test will not have an allergic reaction.25 The bottom line is as follows. Do not perform skin testing unless antivenom is to be given; do not use for dry bites, as the patient possibly will be sensitized unnecessarily. Also, do not waste time with skin testing in cases of severe envenomations. Be prepared to manage anaphylaxis in any patient when antivenom is given regardless of skin test results. If time permits, pretreat the patient with at least 1 liter crystalloid, H1 (diphenhydramine 25-50 mg IVP) and H2 (ranitidine 50 mg IVP) blockers before antivenom is given.
The initial dose of polyvalent antivenom is 10 vials, but some species (Eastern diamondback) usually are given higher doses (10-20 vials). Hemodynamically unstable patients should be started with 20 vials. Be aware that one must reconstitute the antivenom and that bottles must be handled gently to avoid denaturing the IgG. Do not shake vials to dissolve the antivenom, only swirl the solution. It will take 20-30 minutes to reconstitute each vial, so be sure to reconstitute all the vials simultaneously. Add the vials to 250 or 500 mL of 5% dextrose in water or normal saline. Pediatric patients should have their antivenom reconstituted in 20-40 mL/kg of normal saline (up to 1 liter). Start the infusion slowly for the first 10 minutes and if no complications develop, finish the infusion in 1 hour. Patients who have had a positive skin test reaction (wheal at injection site) should have initial doses given even more slowly, and the initial dose should be completed in 2 hours instead of 1 hour. Treat anaphylaxis or systemic reactions by stopping the infusion and treating with epinephrine and additional diphenhydramine, steroids, and ranitidine. If systemic envenomation symptoms are life-threatening, the antivenom should be restarted by further dilution and slowing the infusion. It can be a difficult decision to proceed with antivenom treatment in patients who also have manifested signs of significant allergic reaction, and these cases must be decided on an individual basis. Strongly consider prophylactic intubation in patients when significant allergic reactions develop. If symptoms continue to progress despite antivenom treatment, the initial dose can be repeated in about 1-2 hours. This process continues until the envenomation symptoms stabilize.
CroFab. CroFab was approved by the FDA in October 2000 and is produced from sheep exposed to the venom of four different North American snakes: the Eastern and Western diamondback, the cottonmouth, and type A Mojave rattlesnakes. Four different groups of sheep are given one type of venom each, and IgG is collected and pooled from each. The IgG is further modified into Fc and Fab fragments. The Fab fragments are isolated using affinity chromatography against the venom for which they were developed. These Fab fragments are a purer version of the polyvalent antivenom and are less immunogenic. Also being a smaller molecule, the Fab fragments better penetrate tissues and are cleared more easily by the kidneys. Animal studies find that CroFab is 3-10 times more potent than the polyvalent antivenom.26 Although the manufacturer suggests use for mild and moderate envenomations, it also is used clinically for severe envenomations with no reported problems. There is a hotline to call for any questions on CroFab use: 87-SERPDRUG or 877-377-3784.
Dosing for CroFab is significantly different than for polyvalent antivenom. Skin testing is not recommended for CroFab, and prophylaxis for allergic reactions is not standard either. The initial starting dose is only 4-6 vials diluted in 250 mL of normal saline. Reconstituting CroFab still takes between 20 and 30 minutes, and the vial should never be shaken as activity of the antivenom will be lost in the process. As with the polyvalent antivenom, start the initial infusion slowly for the first 10 minutes and then give the remainder in 1 hour if no allergic symptoms appear. The patient is observed for another hour, and if symptoms progress an additional 4-6 vials are given every 2 hours until stabilization occurs. Reports show that most patients are stabilized with 8-12 vials.4 The package insert and some clinicians recommend that after stabilization of symptoms, 2 vials should be given every 6 hours for a total of 3 additional doses to prevent re-occurrence of symptoms. The efficacy of these extra doses still is debated and is not advised by some toxicologists. CroFab has been used in pediatric cases with success similar to adult cases.27 Be aware the coagulopathies, secondary to thrombocytopenia, have been reported to reoccur up to 2-3 weeks after CroFab treatment,28 and some authors recommend bringing the patient back for recheck during that time. The mechanism of this thrombocytopenia is unclear.
Lastly, although polyvalent antivenom was only produced from two North American rattlesnakes (and two Central or South American species), it was found to be active against all venomous vipers in North, Central, and South America. Apparently, CroFab may have similar cross-reactivity. Initial studies of CroFab use for copperhead and Southern Pacific rattlesnakes show CroFab also is effective for envenomations by these snakes.29,30
Coral Snakes. Treatment of coral snake bites is very different than for any other native venomous snakebite: Antivenom treatment is to begin immediately for any confirmed Eastern or Texas coral snakebite. Again, antivenom treatment is not anticipated for the Sonoran or Arizona coral snake. (See section on Coral Snakes.) Further, antivenom manufactured by Wyeth-Ayerst (Antivenin Micrurus fulvius) is made using only Eastern coral snake venom and has no proven benefit against Sonoran coral snake venom. Future production of coral snake antivenom by the manufacturer is in question, but as of early 2005 emergency supplies are still available. As previously mentioned, once the neurologic complications of coral snake envenomation appear they may not respond to antivenom treatment. Patients with respiratory distress should be treated aggressively and intubated early in the course of treatment. Since coral snake venom is neurotoxic, very few if any local symptoms may be present. Again, the grading scale in Table 1 is not to be used for coral snakebite evaluation. Resist the temptation to underestimate the potential risk to these patients. If a patient has been bitten by an Eastern coral snake, or snakebite is strongly suspected, 4-6 vials of coral snake antivenom (Antivenin Micrurus fulvius) should be given immediately, and the patient should be admitted for observation. If any symptoms develop while in observation, an additional 10 vials may be required, and the patient should be monitored in an ICU setting for possible respiratory failure. Patients must be examined closely for any neurologic deficits; ptosis or other cranial nerve abnormalities may be subtle.
There is no CroFab-type antivenom for coral snakes, only the older type polyvalent antivenom made from horse serum is available. Thus, treatment should be similar to polyvalent-type antivenom used for pit viper bites. (See previous section on Antivenom.) Though recommended by the manufacturer, skin testing is not indicated as it is an inaccurate way to predict possible allergic reactions, and antivenom will be given for any envenomations.7 Pretreat the patient with at least 1 liter crystalloid, H1 (diphenhydramine 25-50 mg IVP) and H2 (ranitidine 50 mg IVPB) blockers. Start with 4-6 vials diluted in 500-1000 mL (20 mL/kg for pediatric patients).7 Infuse the antivenom slowly at first, and the physician should remain at bedside for at least 15 minutes to watch closely for signs of allergic reaction. If no reactions occur, infuse the entire dose in 2 hours. If symptoms continue, an additional 3-5 vials are given. Most envenomations do not require more than 10 vials. Epinephrine, steroids, and additional antihistamines should be given for any signs of allergic reaction, and antivenom infusion halted. After the reaction is treated, one must decide whether to continue the infusion or treat the envenomation with supportive care only. Keep in mind that respiratory paralysis after coral snakebite has been known to take anywhere from days to weeks of mechanical ventilation.11 Again, all patients treated for coral snakebites should be admitted to an ICU setting, even in the case of a dry bite. As mentioned previously, symptom onset can be delayed 12 hours and may begin with respiratory distress. Unfortunately, the manufacturer may discontinue antivenom production in the near future, and physicians will be left only with supportive care until another company produces antivenom.
Exotic Snakes. Treatment of victims of exotic venomous snakebite will depend entirely on the species of snake involved. This section will present a brief overview of symptoms and treatment of the snakes most likely to be encountered. Remember, one must identify the snake to determine what effects are likely, if antivenom for this species exists, and where it may be obtained. The local zoo often is a good resource for identification of the species and for exotic snake antivenom as any good herpetologist will keep his or her own supply of appropriate antivenom when keeping exotic venomous snakes. One also can call the national poison control center number to speak with a toxicologist (800-222-1222) that automatically connects to the nearest poison control center. The Arizona Poison and Drug Information Center (520-626-6016) also is a good back-up resource for venomous snakebite treatment and maintains a database on antivenom stocks in the United States. There is an antivenom index maintained by the American Zoo and Aquarium Association (www.aza.org) that contains valuable data as well. It includes a list of medically important venomous snakes, recommendations for which antivenom to use in treatment, where these antivenoms can be found in the United States, and a list of antivenom manufacturers around the world. This index must be purchased in advance or one must be a member to log into the index online.
Cobras are probably the most likely exotic snake to bite and cause a patient to present for treatment in the United States. As mentioned previously, one series found that cobras were responsible for 40% of non-native snakebites treated in the United States.19 They are very popular in the pet trade, and to many herpetologists they represent the quintessential venomous snake. Their neck can be flattened to form the infamous hood that is universally recognized. The king cobra is the largest of all venomous snakes, reaching up to 5 m (18 feet) in length. Cobra venom is among the most potent of all the world’s venomous snakes, and a single bite from an adult king cobra is estimated to deliver enough venom to kill a full-grown elephant or 20 people. There are a variety of cobra species found in Asia, Africa, and Indonesia. This is an important fact because the venom of each species will vary enough that antivenom produced from one species may not be effective for another. In other words, Indian cobra antivenom may not treat a bite from a Chinese cobra or a Pakistani cobra. Further, some cobra venom is neurotoxic (king cobra) while that of other species (spitting cobras) is more locally damaging like that of a rattlesnake. Patients injected with neurotoxic venom present like those with a coral snakebite; few local symptoms but progressive neurologic dysfunction can develop. Spitting cobras also spray venom with unerring accuracy at the eyes, and although it will not be absorbed systemically, the venom will cause corneal ulcers and blindness if not washed away quickly. Cobra venom also contains a cardiotoxin that binds to heart cells and permanently depolarizes their membranes. Baseline laboratory studies similar to those ordered for native pit viper envenomations (CBC, renal function tests, PT/PTT) should be ordered. As with coral snakebites, the pit viper envenomation grading scale (see Table 1) is not to be used. Any patient with a bite from a cobra should be treated as if a serious envenomation has occurred. Be prepared to provide ventilatory support and attempt to locate antivenom as previously described. Mambas (green or black), kraits, sea snakes, and Australian snakes such as the taipan and tiger snake all produce neurotoxic venom and should be treated in a similar fashion to cobra bites. Again, remember that each of these snakes has its own antivenom and antivenom from one species will not treat envenomation of another species.
Vipers commonly seen in the pet trade include the Russell’s viper (Asia), gaboon viper (Africa), puff adder (Africa), eyelash vipers (Mexico, Central America), and Wagler’s viper (Asia). These vipers produce envenomation symptoms similar to North American pit vipers. Patients experience hemorrhage and local necrosis, and significant local pain and swelling. More severe cases will have systemic signs of hypotension, pulmonary edema, and renal failure. Antivenom is available for many of these vipers, but again geographical differences are important. An extreme example is Russell’s viper where antivenom is effective against Russell’s viper from one area but not those from a different geographic region.
Finally, one may be presented with a choice between using a polyvalent antivenom made from venom from several snakes in a region, and using a monovalent antivenom made from only one snake species. The monovalent antivenom always should be used if the snake has been positively identified. Monovalent antivenoms are more effective in clinical use than polyvalent antivenoms as they provide more specific treatment. If in doubt on the identification of the snake, use the polyvalent type of antivenom. Using monovalent antivenom for the wrong species will result in no protection for the patient, but all of the risks from allergic reaction.
Venomous Lizards. Only two venomous lizards exist, and only the Gila monster is native to the southwestern United States. The Mexican beaded lizard is, as the name suggests, found in Mexico. Both are similar in appearance: stocky, with large heads and strong jaws. Venom is similar to that of rattlesnakes, but is not delivered by hypodermic-like teeth. Most bites occur from captive animals rather than attacks in the wild. Gila monsters tend to bite and hold on, chewing to force venom into the wound by capillary action along grooves in the teeth. Envenomation is not extremely effective and only occurs in up to 70% of bites.30
Treatment first consists of removing the animal from the patient, which may not be an easy task. Options include running hot water over the lizard or gently prying the jaws apart with a metal or wooden object. Care must be taken so that the person removing the animal does not also become a bite victim. If envenomated, burning pain and local swelling likely will be present. Systemic reactions with hypotension and tachycardia sometimes also are seen. Electrolytes, renal function, CBC, and coagulation studies should be ordered as in some cases coagulopathies have been reported.30 Anyone with systemic signs of envenomation should be admitted for further observation/treatment. Patients with apparent dry bites (no envenomation) should be observed for 6 hours and may be discharged if no symptoms develop. Bite wounds should be X-rayed as teeth are relatively large and often are found in the wounds. Prophylactic antibiotics are not indicated, but wound rechecks at 24-48 hours should be performed.
Other Reptiles. Attacks by alligators have been increasing in southern Florida as the populations of alligators and humans continue to grow and increase the chances of contact. More than 200 alligator attacks (13 fatalities) have occurred in Florida since 1948, and 130 of these have been in the past 20 years.32 Bites from these animals can be very dangerous as they have very strong jaws, and can reach lengths of more than 14 feet and can weigh more than 1000 pounds. Attacks tend to occur when the victim is in the water or at the water’s edge. Alligators can approach the shore, remaining hidden in relatively shallow water until the last second when they erupt out of the water, grab the victim, and pull him or her under water. Victims of large alligator bites likely will be multi-trauma patients, but wounds from relatively small animals may be treated by the ED physician alone. Wounds are prone to infection and must be debrided. One study found Aeromonas hydrophila and Clostridium species in alligator mouth cultures,33 and prophylactic antibiotics should include ciprofloxacin to cover these pathogens. Salt-water crocodiles are native to the southern tip of Florida and rarely encounter people. Bite wounds would be treated in a similar manner to alligator bites.
A variety of turtles are native to the United States, and most do not bite humans. Even if they do, significant injury does not result. Exceptions include the common snapping turtle and the alligator snapping turtle of the southeastern United States. Common snapping turtles grow large enough (up to 30 pounds) to cause substantial finger injuries, and alligator snapping turtles grow large enough (more than 200 pounds) to threaten entire limbs. These animals have very strong jaws but no teeth. One might only see a laceration on the surface, but X-rays should be taken to assess for possible fractures. Healthy, low-risk patients usually do not require prophylactic antibiotics for minor wounds, but wounds should be left open. Open fractures should be treated as contaminated and referred to the orthopedist.
Marine Animals
Marine Animal Bites. All fish can bite, but few are large enough to produce any significant damage. Most bites that require treatment will occur in scuba divers or fishermen. In general, bites from fish can be treated with good local wound care in a similar manner to any bite wound. Most bites will be small puncture wounds that will not require sutures. Primary closure of marine bite wounds can follow the same recommendations as for animal bites: only disfiguring, low-risk (facial) wounds are usually closed, and higher risk wounds (hands) will be left open. Larger bites can occur from larger fish (i.e., sharks, barracudas, and moray eels). As with all traumatic wounds, tetanus prophylaxis is indicated if the patient is not already up to date on immunizations.
Human attacks on sharks outnumber attacks of sharks on people by at least 10 million to 1.34 Still, shark bites garner an extraordinary amount of attention when they do occur. Injuries can vary from minor with smaller species, to life-threatening with large sharks. Most people appear to be bitten out of mistaken identity where the shark encounters the person in the water, bites once, and then swims away after recognizing the victim as not a normal food source. Unfortunately, this inquisitive bite may be life-threatening in itself. Major shark bite victims often are in hemorrhagic shock from blood loss and should be managed according to standard trauma assessment and resuscitation procedures. Prophylactic antibiotics are indicated as is tetanus prophylaxis. Barracudas are attracted to shiny objects, which are assumed to simulate flashing scales of a struggling fish. Barracudas produce V-shaped lacerations as compared to the crescent-shaped lacerations of shark bites, and can be treated in a similar fashion. Moray eels often bite hands of divers or snorklers when they reach into a hole in the reef. They make several smaller puncture wounds with their teeth, but as they tend to hold on after biting may also cause lacerations if pulled forcibly off a victim. Moray eel bites are high risk for infection and should be treated prophylactically in every case.
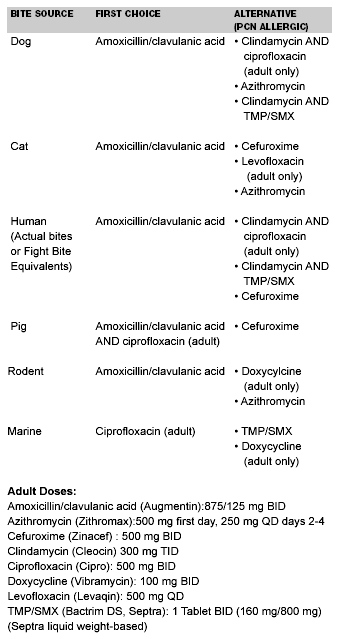
Wound infections can occur from a variety of marine bacteria, and freshwater and saltwater bacteria vary widely. Notable saltwater pathogens include various Vibrio species which are gram-negative rods that seem to grow exceptionally well in human tissue. Certain species can be highly virulent. For example, Vibrio vulnificus can produce sepsis, necrotizing fasciitis, meningitis, and endocarditis with a fatality rate near 30%.34 Likewise, freshwater Aeromonas species also can cause aggressive soft-tissue infections. General prophylaxis recommendations are as follows. Small wounds in normally healthy patients do not require prophylaxis. Immunocompromised patients or larger wounds that require surgical repair or hospitalization should be treated prophylactically. (See Table 3.) Ciprofloxacin (Cirpo 500 mg PO BID) is the first choice, followed by trimethoprim/sulfamethoxazole (Bactrim 1 DS PO BID) and doxycycline (Vibramycin 100 mg PO BID).
Table 3. Recommendations for Prophylactic
Antibiotics Treating Animal Bites (5-Day Course)51-53
Marine Envenomations. Jellyfish. Jellyfish are found in all oceans, and of the nearly 10,000 species of jellyfish, only about 100 are known to be dangerous to humans.34 Of these 100, only two are potentially deadly: the Portuguese man-of-war and the box jellyfish. Deaths from the man-of-war are relatively rare and may be related to complications of anaphylaxis rather than directly from envenomation. Box jellyfish venom is quite toxic, with deaths occurring yearly before antivenom was developed. Fortunately box jellyfish are found only in Australian waters, but the man-of-war is native to southern U.S. waters. All jellyfish have some variation of stinging tentacles that hang down from a central bell or body. The tentacles contain stinging cells (nematocysts) that discharge a small sharp thread that penetrates the skin and facilitates envenomation. Any contact with the tentacle triggers discharge of the nematocysts. Millions of nematocysts can be found on a single tentacle, and some jellyfish have tentacles more than 10 feet long. Unfortunately, nematocysts are hardy and can envenomate long after the animal’s death and hours after being washed onto a beach. Jellyfish venom is designed to incapacitate fish rapidly, as the fragile bodies of the jellyfish easily would be damaged by struggling prey. Analysis of jellyfish venom has been difficult to perform, and the only antivenom produced is for the box jellyfish.
Treatment of jellyfish stings is along two fronts: minimize further envenomation and treat existing symptoms. Many patients exit the water with tentacles still attached to their skin. In all patients, these tentacles must be removed to prevent further envenomation. Rubbing the skin to remove adhered tentacles or washing with hot water likely will worsen discharge of remaining nematocysts and should be avoided. The best technique for most jellyfish is to apply 5% acetic acid (vinegar) or isopropyl alcohol to the wound for up to 30 minutes to deactivate the nematocysts. If vinegar is unavailable, some authors recommend fresh urine as an alternative. Otherwise, rinse the area with saltwater and gently remove tentacles with forceps or gloved hands. Simple surgical gloves usually are enough protection for the caregiver.
Envenomation can be mild or severe. Symptoms of mild envenomation are limited to local dermatitis that can persist for several weeks, depending on the severity. In more significant envenomation, mild skin necrosis and ulceration can be seen. These injuries usually are significant only if they occur on the face or in the eyes (keratitis and corneal ulcers). Severe envenomations produce more dramatic local reactions and systemic symptoms that appear quickly or in several hours depending on the health of the patient and the extent of the envenomation. Systemic effects can be quite varied and can affect every organ system.35 Neurological complications include headache, vertigo, ataxia, paralysis, peripheral nerve palsies, delirium, seizures, coma, and death. Cardiovascular complications include anaphylaxis, hemolysis, hypotension, dysrhythmias, vascular spasm, deep venous thrombosis, congestive heart failure, and ventricular fibrillation. Respiratory complications include bronchospasm, laryngeal edema, pulmonary edema, and respiratory failure. Other effects include muscle spasm, fat atrophy, arthralgias, reactive arthritis, vomiting, diarrhea, dysphagia, hypersalivation, and acute renal failure.
Treatment of systemic complications is supportive as only the box jellyfish (Australian species) has antivenom available (CSL Box Jellyfish Antivenom). Note that like anaphylaxis, most patients will be admitted for continued treatment but some will recover to baseline in the ED. Any patient with severe symptoms who recovers in the ED must be observed for at least 6-8 hours to watch for rebound of symptoms. Hypotension primarily is seen in the very young or old, or in cases of contact with multiple tentacles. Resuscitation with IV fluids usually is enough, but vasopressors may be required. An ECG, CBC, electrolytes, renal function tests, and UA should be ordered to evaluate for dysrhythmias, hemolysis, and renal failure. Be aware that regional arterial spasm may be refractory to all treatment, and watch for compartment syndromes. Opiates often will be needed to help with pain control. Early consultation with a toxicologist is suggested for help treating systemic symptoms.
Fire Coral. While not a true coral, fire coral does resemble hard corals and grows in similar locations. Many nematocysts (stinging cells) are contained on its surface, and immediately after contact with skin, a strong burning sensation develops. Itching of the area will further exacerbate the envenomation, but even so symptoms usually fade within 60-90 minutes. Treatment consists of rinsing the area with isopropyl alcohol or dilute vinegar to denature the nematocysts and reduce further envenomation.
Sea Urchins. Sea urchins are found in both cold water and tropical environments on the entire U.S. coast. Sea urchins are relatively small (less than 1 foot in diameter) echinoderms that all share a similar body design. They have a hard, roundish body covered in spines. The spines are used both for defense and locomotion. They are very slow moving, and most people either step on the spines or bump into them while swimming or diving. The spines vary in length from less than 1 inch to nearly 12 inches, and can be venom-bearing as well. The spines can be extremely sharp, easily penetrating wet suits, and can be lodged deep into tissues. Thus the two main problems from sea urchin encounters are envenomation and presence of a foreign body.
Envenomation usually occurs via puncture from the spines and symptoms are immediate, intense, burning pain at the site followed by redness and swelling. If several spines have entered the patient systemic symptoms may follow, including local muscle pain, vomiting, paresthesias, muscle paralysis, abdominal pain, hypotension, and respiratory distress.34 Fortunately envenomations rarely are fatal. No antivenom exists, and treatment consists of immersion of the body part in non-scalding hot water (less than 45°C or 113°F) to help reduce pain.
Spines are composed of calcium carbonate and silica and very thin ones will reabsorb over days to several weeks. Thicker spines should be removed as they will not reabsorb and are a potential nidus of infection. Spines have been documented to enter joints and may not show up with plain X-rays. Customary treatment from local fishermen consists of massage or striking a retained spine to break it up and speed absorption, but is not recommended. Unless a spine easily can be plucked from the surface, removal is best done by a surgeon using an operating microscope to take out all of the spine pieces. Magnetic resonance imaging (MRI) may be required to accurately locate retained spines, but ultrasound also may be useful in the ED. Bacterial infections are common after such puncture wound injuries and should be treated with prophylactic antibiotics. (See Table 3.) Injuries often occur to the plantar surface of the foot, and should be considered for referral to the consultant for follow-up after evaluation in the ED.
Octopus. Octopus and cuttlefish normally are not dangerous to humans. However, if handled or captured, they can bite with a parrot-shaped beak located in the center of the tentacles. As most octopus have small beaks, all envenomations can be avoided by wearing gloves. Only the blue-ring octopus native to the Indo-pacific is known to be deadly to humans. Envenomation from Caribbean octopus species can cause some mild systemic symptoms (i.e., nausea, vomiting, fever, paresthesias), but no antivenom exists for any octopus venom.
Stingrays. There are 22 species of stingray found in U.S. coastal waters.34 While they are related to sharks, stingrays do not cause problems through use of their teeth. Stingrays have from 1-4 venomous spines on their tails that they use for self-defense. Stingrays usually are encountered by humans in shallow sandy or muddy patches. Injury occurs when a person accidentally steps on the partially buried stingray or during improper handling of the animal. The animal whips its tail upward and drives the spine into the person, and venom is released in the process. The injury is either a puncture wound or jagged laceration. In some cases, the tip of the spine breaks off into the wound.
Symptoms of envenomation include local pain and swelling as well as systemic reactions varying from vomiting, diarrhea, vertigo, headache, syncope, and seizures.34 Deaths are rare, but have been reported due to hypotension and arrhythmias. As with sea urchins and stinging fish, treatment consists of placing the affected limb in non-scalding hot water (less than 45°C or 113°F) to help reduce pain. Pain usually peaks in fewer than 60 minutes and resolves in fewer than 48 hours. The wound should be explored to remove any retained parts of the spine or its covering. Be aware that stingrays are cartilaginous fish without bones, therefore their spines may not show up on plain films. The wound should be debrided thoroughly, left open, and packed with gauze. Prophylactic antibiotics are recommended as protracted infections are common and even can lead to osteomyelitis.
Stinging Fish. Catfish are found both in fresh and salt water. Catfish derive their name from the sensory whiskers around their mouths, but it is the spines of the dorsal and pectoral fins that injure. People are injured when handling the fish, usually in an attempt to remove it from a fishhook. Envenomation from the catfish spine produces a puncture wound with associated throbbing or burning pain radiating up the extremity. Pain usually lessens in 30-60 minutes, but can last for 2 days. As with stingrays, the spines of catfish can break off in the wound. Systemic effects (i.e., lymphedema, weakness, hypotension, and respiratory distress) are much less common, and death is very rare. Salt water species usually produce more pronounced symptoms.
Like catfish, stonefish, scorpion fish, and lion fish all have venomous spines on their dorsal and/or pectoral fins. Stonefish are named because they sit motionless on the sea floor and mimic a rock. Stonefish are not native to North American waters but are found in Hawaii. People characteristically are injured as they step on the motionless fish. Scorpion fish are similar to stonefish and rely on camouflage to conceal themselves on the ocean floor. They are native to U.S. waters, but are found only in Gulf coast states, California, and Hawaii. People also are injured by stepping on these fish. Lion fish are related, but are free-swimming and brightly colored to stand out from their surroundings. While these fish are not native to the United States, as early as 2000 they began to be reported seen off the U.S. coast, likely from release of imported fish. They also are popular with saltwater aquarium enthusiasts and injure their keepers when improperly handled.
Stonefish and scorpion fish envenomations produce the most severe symptoms with local pain and some tissue destruction. Local pain may persist for weeks, and ulcers may take months to heal. Systemic effects can include headache, tremors, maculopapular skin rash, vomiting, diarrhea, abdominal pain, diaphoresis, pallor, delirium, seizures, limb paralysis, peripheral neuropathy, arthritis, fever, hypertension, respiratory distress, dysrhythmias (bradycardia, tachycardia, atrioventricular block, ventricular fibrillation), congestive heart failure with pulmonary edema, pericarditis, hypotension, and death.34 Lion fish produce less severe systemic reactions.
Treatment for all stinging fish is similar. The affected limb should be immersed as soon as possible in non-scalding hot water (less than 45°C or 113°F) to help reduce pain. Repeat immersion may be indicated if pain returns in 1-2 hours after initial treatment. Wounds are explored to ensure removal of any retained spines or tissue and left open. Hand or foot wounds should be treated with prophylactic antibiotics. Antivenom only exists for stonefish venom, and those practicing in Hawaii are referred to reference text for its use.34
The Pregnant Patient
Pregnant patients represent a unique subset of patients and require careful consideration of potential risks and benefits for both mother and fetus during evaluation and treatment in the ED. Patients always are concerned for their own outcome, but adding fears of how the patient’s treatment will affect their unborn child add an entirely new dimension. Fortunately, the adage that "what is good for the mother is good for the baby" also applies to treatment of pregnant patients suffering from bites and stings. The next section briefly will review some of the more serious envenomations: pit vipers and the black widow spider. For a detailed review, see the recent review of the current literature by Langley.36
Venomous Snakes. Envenomation from a venomous reptile can be taxing enough, but treating a pregnant victim is potentially more so as there are now two patients to care for. Fortunately, venomous snakebite during pregnancy is a rare event, but the consequence also is that much less is known about fetal effects. There is one old case report linking fetal malformation with snakebite,37 along with several more recent case reports. One documents an intrauterine death,38 one describes placental abruption,39 and the other premature labor and delivery without fetal complication.40 A complete review of the literature from 1966 to 2002 found only 85 cases of snakebite documented during pregnancy.36
As the majority of venomous snakebites in the United States are from pit vipers, the same is true for pregnant patients. Severe hypotension and coagulation disorders are two of the more dangerous complications that can place both the mother and fetus at risk. One review of pit viper envenomation in pregnancy found a disturbingly high rate of complication: an overall 43% rate of fetal wastage, and a maternal mortality of 10%.41 These complications give the patient a severe envenomation categorization (see Table 1) and indicate antivenom therapy. While the FDA designates antivenom category C or uncertain safety—animal studies show no adverse effects, and there are no human studies. Promethazine (Phenergan) and nifedipine (Procardia) are two commonly used drugs in pregnancy that also are FDA category C drugs. Hypotension and coagulopathy complications represent a very real threat to the patient’s life, and antivenom treatment should not be withheld because of pregnancy (except in the case of a competent patient refusing treatment). At least one paper supports that antivenom and other antidotes not formally studied should be used when the mother’s life is in danger.42 A final point concerning treatment of hypotension comes from a case report in Australia where a young brown snake (neurotoxic venom) bit a patient near term. The patient died, and autopsy results suggested envenomation was not severe enough to cause death; rather supine hypotensive syndrome was to blame.43 Thus, remember to keep pregnant patients in the third trimester on their left side to avoid exacerbation of positional hypotension via compression of the inferior vena cava by the gravid uterus.
Black Widow Spider. Black widow spider bites are not common in pregnancy but do occur and can be problematic as they can mimic preeclampsia or abruption, with hypertension, acute abdominal pain, headache, and proteinuria.44 There are four case reports in the literature and in all cases there were no fetal complications.44-47 In two cases the patients received antivenom with no apparent ill effects and successful treatment of envenomation symptoms.46,47 As most patients recall the spider bite and can assist in identification, one should remember to ask about spider bite in unclear cases of acute abdominal pain in pregnancy.
Other Bites and Stings. Bees and wasps can produce anaphylaxis in pregnant patients as well as non-pregnant patients. Treatment of anaphylaxis is the same for pregnant patients, with emphasis on airway management, correction of hypotension, epinephrine, diphenhydramine, and corticosteroids. All of these medicines have been used in treatment of anaphylaxis in pregnancy without evidence of harm. There is even a case report of a woman developing anaphylaxis during labor from ampicillin given for Group B streptococci, who successfully delivered a healthy baby while on an epinephrine drip.48 There are no case reports in the literature on bark scorpion stings in pregnant patients. Most of the severe reactions occur in children, and treatment is supportive in any case as no antivenom is commercially available. Animal and marine animal bites are treated the same in pregnant or non-pregnant patients. Both the tetanus toxoid49 and rabies post-exposure prophylaxis are considered safe in pregnancy.50
References
1. Weed HG. Nonvenomous snakebite in Massachusetts: Prophylactic antibiotics are unnecessary. Ann Emerg Med 1993;22:220-224.
2. Blaylock RS. Antibiotic use and infection in snakebite victims. S Afr Med J 1999;89;874-876.
3. Watson WA, Litovitz TL, Rodgers GC Jr, et al. 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2003;21:353-421.
4. Gold, BS, Barish RA, Dart RC. North American snake envenomation: Diagnosis, treatment and management. Emerg Med Clinics N Amer 2004;22: 423-443.
5. Chippaux JP. Snake-bites: Appraisal of the global situation. Bull World Health Organ 1998;76:515-524.
6. Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment.West J Med 1988;148:37-44.
7. Norris RL, Bush S. North American venomous reptile bites. In: Auerbach PS, ed. Wilderness Medicine, 4th ed. St. Louis: Mosby Inc;2001:896-926.
8. Gopalakrishnakone P, Hagwood BJ, Holbrooke SE, et al. Sites of action of Mojave toxin isolated from the venom of the Mojave rattlesnake. Br J Pharmacol 1980;69:421-431.
9. Parrish HM. Incidence of treated snakebites in the United States. Public Health Rep 1966;81:269-275.
10. Whitley RE. Conservative treatment of copperhead snakebites without antivenin. J Trauma 1996;41:219-221.
11. Kitchens CS, Van Mierop LHS. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): A study of 39 victims. JAMA 1987;258: 1615-1618.
12. Kitchens CS. Hemostatic aspects of envenomation by North American snakes. Hematol Oncol Clin North Am 1992;6:1189-1195.
13. Fix JD. Venom yield of the North American coral snake and its clinical significance. South Med J 1980;73:737-738.
14. Wingert WA, Wainschel J. Diagnosis and management of envenomation by poisonous snakes. South Med J 1975;68:1015-1026.
15. Gold BS, Wingert WA. Snake venom poisoning in the United States: A review of therapeutic practice. South Med J 1994;87:579-589.
16. Norris RL, Minton SA. Non-North American venomous reptile bites. In: Auerbach PS, ed. Wilderness Medicine, 4th ed. St. Louis: Mosby Inc; 2001: 927-951.
17. Jenkins M, Russell FE. Physical therapy for snake venom poisoning. Phys Ther 1974;54:1298-1304.
18. Russell FE. AIDS, cancer, and snakebite-what do these three have in common? West J Med 1988;148:84-89.
19. Minton SA. Bites by non-native venomous snakes in the United States. Wilderness Environ Med 1996;7:297-303.
20. de Haro L, Pommier P. Envenomation: A real risk of keeping exotic house pets. Vet Hum Toxicol 2003;45:214-216.
21. Silveria PV, Nishioka C de A. Non-Venomous snake bite and snake bite without envenoming in a Brazilian teaching hospital. Analysis of 91 cases. Rev Inst Med Trop Sao Paulo 1992;34:499-503.
22. Davidson TM, Schafer SF. Rattlesnake bites. Guidelines for aggressive treatment. Postgrad Med 1994;96:107-114.
23. Horowitz RS, Dart RC. Anitvenins and immunobiologicals: Immunotherapeutics of envenomation. In: Auerbach PS, ed. Wilderness Medicine, 4th ed. St. Louis: Mosby Inc; 2001: 952-960.
24. Sullivan JB. Past, present, and future immunotherapy of snake venom poisoning. Ann Emerg Med 1987;16:938-944.
25. Jurkovich GJ, Luterman A, McCullar K, et al. Complications of Crotalidae antivenin therapy. J Trauma 1988;28:1032-1037.
26. Consroe P, Egen NB, Russell FE, et al. Comparison of a new ovine antigen binding fragment (Fab) antivenin for United States Crotalidae with the commercial antivenin for protection against venom-induced lethality in mice. Am J Trop Med Hyg 1995;53:507-510.
27. Offerman SR, Bush SP, Moynihan JA, et al. Crotaline Fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics 2002;110: 968-971.
28. Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med 2001;37:196-201.
29. Lavonas EJ, Gerado CJ, O’Malley G, et al. Initial experience with Crotalidae polyvalent immune Fab (ovine) antivenom in the treatment of copperhead snakebite. Ann Emerg Med 2004;43:200-206.
30. Bush SP, Green SM, Moynihan JA, et al. Crotalidae polyvalent immune Fab (ovine) antivenom is efficacious for envenomations by Southern Pacific rattlesnakes (Crotalus helleri). Ann Emerg Med 2002;40:619-624.
31. Strimple PD, Tomasssoni AJ, Otten EJ, et al. Report on envenomation by a Gila monster (Heloderma suspectum) with a discussion of venom apparatus, clinical findings, and treatment. Wilderness Environ Med 1997;8:111-116.
32. Burgess GH, Callahan MT, Howard RJ. Sharks, alligators, barracudas, and other biting animals in Florida waters. J Fla Med Assoc 1997;84:428-432.
33. Flandry F, Lisecki EJ, Dominique GJ, et al. Initial antibiotic therapy for alligator bites: Characterization of the oral flora of Alligator mississippiensis. South Med J 1989;82:262-266.
34. Auerbach PS. Envenomation by aquatic invertebrates. In: Auerbach PS, ed. Wilderness Medicine, 4th ed. St. Louis: Mosby Inc; 2001: 1450-1487.
35. de Freitas JC, Schiozer WA, Malpezzi EL. A case of envenoming by Portuguese man-of-war from the Brazilian coast. Toxicon 1995;33:859-862.
36. Langley RL. A review of venomous animal bites and stings in pregnant patients. Wilderness Environ Med 2004;15:207-215.
37. Malz S. Snakebite in pregnancy. J Obstet Gynaecol Br Commonw 1967; 74:935.
38. Nasu K, Ueda T, Miyakawa I. Intrauterine fetal death caused by pit viper venom poisoning in early pregnancy. Gynecol Obstet Invest 2004;57: 114-116.
39. Zugaib M, de Barros AC, Bittar RE, et al. Abruptio placentae following snake bite. Am J Obstet Gynecol 1985;151:754-755.
40. McNally SL, Reitz CJ. Victims of snakebite. A 5-year study at Shongwe Hospital, Kangwane, 1978-1982. S Afr Med J 1987;72:855-860.
41. Dunnihoo DR, Rush BM, Wise RB, et al. Snake bite poisoning in pregnancy: A review of the literature. J Reprod Med 1992;37:653-658.
42. Bailey B. Are there teratogenic risks associated with antidotes used in the acute management of poisoned pregnant women? Birth Defects Res A Clin Mol Teratol 2003;67:133-140.
43. Sutherland SK, Duncan AW, Tibballs J. Death from a snake bite: Associated with the supine hypotensive syndrome of pregnancy. Med J Aust 1982;4: 238-239.
44. Sherman RP, Groll JM, Gonzalez DI, et al. Black widow spider (Latrodectus mactans) envenomation in a term pregnancy. Curr Surg 2000;57:346-348.
45. Scalzone JM, Wells SL. Latrodectus mactans (black widow spider) envenomation: An unusual cause for abdominal pain in pregnancy. Obstet Gynecol 1994;83:830-831.
46. Handel CC, Izquierdo LA, Curet LB. Black widow spider (Latrodectus mactans) bite during pregnancy. West J Med 1994;160:261-262.
47. Russell FE, Marcus P, Streng JA. Black widow spider envenomation during pregnancy. Report of a case. Toxicon 1979;17:188-189.
48. Gei AG, Pacheco LD, Vanhook JW, et al. The use of a continuous infusion of epinephrine for anaphylactic shock during labor. Obstet Gynecol 2003;102: 1332-1335.
49. Sheffield JS, Ramin SM. Tetanus in pregnancy. Am J Perinatol 2004;21: 173-182.
50. Chutivongse S, Wilde H, Benjavongkulchai M, et al. Postexposure rabies vaccination during pregnancy: Effect on 202 women and their infants. Clin Infect Dis 1995;20:818-820.
51. Weber EJ, Callaham M. Mammalian bites. In: Marx J, et al, eds. Emergency Medicine: Concepts and Clinical Practice, 5th ed. St Louis: Mosby Inc; 2002: 774-785.
52. Chen E, Hornig S, Shepard SM, et al. Primary closure of mammalian bites. Acad Emerg Med 2000;7:157-161.
53. Freer L. Bites and injuries inflicted by wild animals. In: Auerbach PS, ed. Wilderness Medicine, 4th ed. St. Louis: Mosby Inc; 2001: 979-1001.
