Ceftazidime-avibactam — Formulary Considerations
Ceftazidime-avibactam is a new beta-lactam/beta-lactamase inhibitor combination approved for the treatment of complicated intra-abdominal infections in combination with metronidazole, and complicated urinary tract infections, including pyelonephritis in patients with limited alternative treatment options. The addition of avibactam to ceftazidime extends its spectrum of activity to include organisms that produce Ambler class A and C beta-lactamases, including AmpC, extended spectrum beta-lactamases (ESBLs), and most notably, Klebsiella pneumoniae carbapenemases (KPCs).
GENERIC NAME: Ceftazidime-avibactam
TRADE NAME: Avycaz™
U.S. FDA APPROVAL DATE: February 25, 2015
SIMILAR DRUGS
Meropenem, imipenem-cilastatin, doripenem, ceftolozane-tazobactam
INDICATIONS1
Treatment of adult patients 18 years or older with the following infections:
- Complicated intra-abdominal infections (cIAI), in combination with metronidazole, caused by Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, K. pneumoniae, Proteus mirabilis, Providencia stuartii, and Pseudomonas aeruginosa.
- Complicated urinary tract infections (cUTI) including pyelonephritis caused by Citrobacter freundii, C. koseri, Enterobacter aerogenes, E. cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus spp, and Pseudomonas aeruginosa.
PHARMACOLOGY1,2
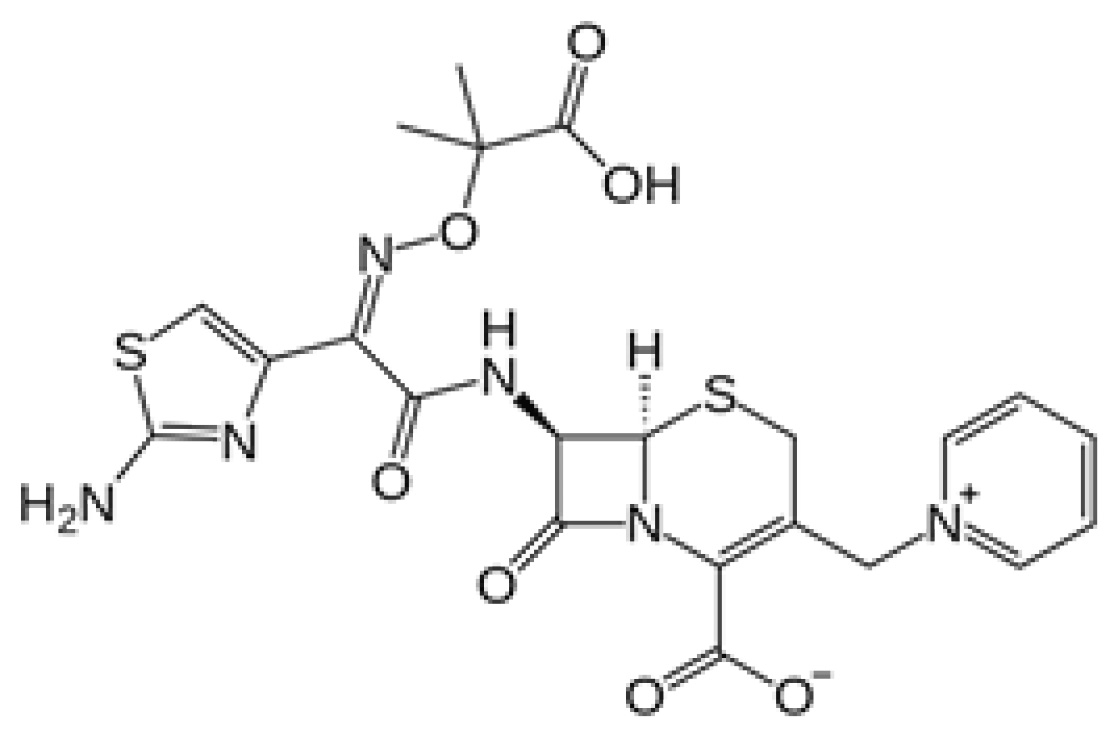
Ceftazidime
Avibactam
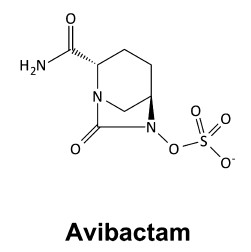
Ceftazidime is a previously FDA-approved, third-generation cephalosporin with broad activity against Gram-negative organisms, including Pseudomonas aeruginosa. It is a bactericidal beta-lactam antibiotic that inhibits cell wall synthesis by binding to penicillin-binding proteins. Avibactam is a novel, non-beta-lactam beta-lactamase inhibitor of Ambler classes A and C beta-lactamases through covalent-binding to the enzyme active site. The addition of avibactam preserves ceftazidime activity by inhibiting its degradation by beta-lactamases and confers enhanced activity against Gram-negative bacteria, including ESBL-producing organisms. More importantly, ceftazidime-avibactam is the only beta-lactam/beta-lactamase inhibitor to date to have activity against KPCs. However, ceftazidime-avibactam has limited activity against Ambler class D beta-lactamases (except OXA-48 carbapenemases) and no activity against Ambler class B metallo-beta-lactamases (MBLs). Additionally, ceftazidime-avibactam has minimal activity against Acinetobacter, anaerobes, and Gram-positive organisms.
PHARMACOKINETICS & PHARMACODYNAMICS1-3
Ceftazidime and avibactam have similar volumes of distribution and half-lives. The volume of distribution of ceftazidime and avibactam is 17 L and 22.2 L, respectively. The half-life of ceftazidime is approximately 3 hours and avibactam is approximately 2.5 hours. Ceftazidime-avibactam dosed at 2.5 g every 8 hours achieves steady-state peak concentrations of ceftazidime and avibactam of 90 and 15 mg/L, respectively. Protein binding is relatively low at less than 10% with ceftazidime and 5.7-8.2% with avibactam. Both are also predominantly excreted renally and removed by hemodialysis. Ceftazidime is minimally metabolized, with 80-90% of the dose being eliminated in urine as unchanged drug. Avibactam is not metabolized, and 97% of the dose is eliminated in urine as unchanged drug.
Similar to the pharmacodynamics of other beta-lactam antibiotics, ceftazidime bactericidal activity is optimized by maximizing the proportion of the time of the dosing interval that the free drug concentration is above the MIC. The suggested pharmacodynamic target of avibactam is the proportion of the time of the dosing interval that the free beta-lactamase inhibitor concentration is above the threshold concentration.
CLINICAL TRIALS/EVIDENCE SUMMARY
- Clinical efficacy and safety of ceftazidime-avibactam is currently limited to Phase 2 studies.
- Results from Phase 3 trials in cUTI and cIAI are completed but have not yet been published. In a Phase 3 cIAI trial, clinical efficacy of ceftazidime-avibactam was decreased in patients with moderate renal impairment (CrCl 30-50 mL/min) compared to patients with normal renal function (CrCl > 50 mL/min).3
CLINICAL TRIALS/EVIDENCE SUMMARY |
|||||||||||||||||||||||||||||||||||
|
Trial |
Population |
Intervention |
Efficacy & Safety Results |
||||||||||||||||||||||||||||||||
|
cUTI = complicated urinary tract infection; cIAI = complicated intra-abdominal infection; MITT = modified intent-to-treat; ME = microbiologically evaluable; CE = clinically evaluable; MR = favorable microbiological response; CR = favorable clinical response |
|||||||||||||||||||||||||||||||||||
|
Vazquez et al.5 Phase 2, multi-center, prospective, double-blind, randomized study |
N = 137 adult patients with cUTI, including pyelonephritis, requiring parenteral |
Ceftazidime-avibactam 500 mg/125 mg IV q8h vs Imipenem-cilastatin 500 mg IV q6h Then step down to oral therapy Duration: 7-14 days total |
|
||||||||||||||||||||||||||||||||
|
Lucasti et al.6 Phase 2, multi-center, prospective, double-blind, randomized study |
N = 204 hospitalized, adult patients with cIAI requiring |
Ceftazidime-avibactam 2.5 g IV q8h + Metronidazole 500 mg IV q8h vs Meropenem 1 g IV q8h Duration: 5-14 days |
|
||||||||||||||||||||||||||||||||
|
*Note ceftazidime-avibactam dose in subgroup with CrCl 30-50 mL/min was 33% lower than is currently recommended. |
||||
|
CrCl > 50 mL/min |
CrCl 30-50 mL/min* |
|||
|
Phase 3 cIAI |
Ceftazidime-avibactam + metronidazole |
Meropenem |
Ceftazidime-avibactam + metronidazole |
Meropenem |
|
Clinical cure |
85% |
86% |
45% |
74% |
|
Mortality |
1% |
1% |
25.8% |
8.6% |
ADVERSE EFFECTS3
- Limited safety data are available at this time, given FDA approval was based on Phase 1 and 2 studies only. Therefore, the manufacturer recommends that ceftazidime-avibactam be reserved for patients who have limited or no alternative treatment options.
- Adverse effects reported at ≥ 10% incidence include nausea, vomiting, constipation, and anxiety.
CONTRAINDICATIONS/WARNINGS/PRECAUTIONS1,3
- Serious hypersensitivity to ceftazidime-avibactam, individual components, or other cephalosporins are considered contraindications. Caution is advised in patients with hypersensitivity to penicillins or carbapenems due to potential for cross-reactivity.
- Neurologic toxicity has been reported with ceftazidime, and the risk is increased in patients with impaired renal function. Symptoms include encephalopathy, myoclonus, seizures, or non-convulsive status epilepticus. Dose adjustment is recommended in patients with renal impairment.
- Similar to other antibiotics, prolonged use may result in superinfection, including Clostridium difficile-associated diarrhea.
- In a Phase 3 cIAI trial, decreased efficacy was reported in patients with moderate renal impairment (CrCl 30-50 mL/min) compared to patients with normal renal function (CrCl > 50 mL/min). Dose adjustment for renal impairment is recommended (see dosage and administration section).
POTENTIAL FOR MEDICATION ERRORS
- Dosing should be stated in total grams rather than grams of individual components to avoid dosing errors.
DRUG INTERACTIONS1,4
- Organic anion transporters (OAT) inhibitors (e.g., probenecid) can decrease elimination of avibactam, and their concurrent use with ceftazidime-avibactam should be avoided.
DOSAGE AND ADMINISTRATION3,4
Recommended dose/duration:
- Dosing is expressed as total grams of the ceftazidime-avibactam combination in a ratio of 4:1, i.e., 2.5 g is 2 g ceftazidime and 0.5 g avibactam.
|
Table. Dosing |
||
|
Indication |
Dose |
Duration |
|
cIAI |
2.5 g IV every 8 hours in combination with metronidazole |
5-14 days |
|
cUTI, including pyelonephritis |
2.5 g IV every 8 hours |
7-14 days |
- Patients with renal impairment should have doses adjusted based on creatinine clearance as calculated by the Cockcroft-Gault equation.
|
Estimated Creatinine |
Recommended Dose |
|
HD = hemodialysis |
|
|
> 50 |
2.5 g (2 g/0.5 g) every 8 hours |
|
31-50 |
1.25 g (1 g/0.25 g) every 8 hours |
|
16-30 |
0.94 g (0.75 g/0.19 g) every 12 hours |
|
6-15* |
0.94 g (0.75 g/0.19 g) every 24 hours |
|
≤ 5* |
0.94 g (0.75 g/0.19 g) every 48 hours |
|
*ESRD on HD |
Administer after HD on HD days |
- Both ceftazidime and avibactam are removed by dialysis. Following a 4-hour dialysis session, 55% of the dose was recovered in the dialysate.
- Dose adjustment for hepatic function is not necessary.
- Administration instructions:
- Administer as an intermittent intravenous infusion over 2 hours.
Cost |
||||
|
How Supplied |
Average Wholesale Price (per vial) |
Usual Dose |
Cost of Therapy per day |
|
|
Ceftazidime-avibactam |
2.5 g vials |
$342 |
2.5 g q8h |
$1026.00 |
|
Ceftolozane-tazobactam |
1.5 g vials |
$99.60 |
1.5 g q8h |
$298.80 |
|
Meropenem |
1 g vials |
$18.48 |
1 g q8h |
$55.44 |
CONCLUSIONS
The addition of avibactam to ceftazidime retains ceftazidime’s spectrum of activity and has added activity against some ESBL- and KPC-producing pathogens. Expedited FDA approval of ceftazidime-avibactam was granted based on two small Phase 2 trials and the previously established efficacy and safety of ceftazidime alone. In Phase 2 trials, ceftazidime-avibactam showed similar efficacy to active comparators and appeared to be well-tolerated when used to treat hospitalized adult patients with cUTI and cIAI in combination with metronidazole.
Currently, ceftazidime-avibactam is the only beta-lactam/beta-lactamase inhibitor to have activity against KPCs. In a climate of increasing carbapenem resistance, ceftazidime-avibactam represents an important treatment option in the management of multidrug-resistant Gram-negative infections. With ongoing Phase 3 trials, the precise role of ceftazidime-avibactam remains to be determined and will need to be weighed against the risk of developing drug-resistant bacteria.
REFERENCES
- Lexi-Comp, Inc. (Lexi-DrugsTM). Lexi-Comp, Inc.
- Zasowski EJ, et al. The beta-lactams strike back: Ceftazidime-avibactam. Pharmacotherapy 2015;35:755-770.
- AvycazTM [Package Insert]. Actavis, Inc. 2015.
- Liscio JL, et al. Ceftolozane/tazobactam and ceftazidime/avibactam: Two novel ß-lactam/ß-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections. Int J Antimicrob Agents 2015;46:266-271.
- Vazquez JA, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: Results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 2012;28:1921-1931.
- Lucasti C, et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: Results of a randomized, double-blind, Phase II trial. J Antimicrob Chemother 2013;68:1183-1192.
Ceftazidime-avibactam is a new beta-lactam/beta-lactamase inhibitor combination approved for the treatment of complicated intra-abdominal infections in combination with metronidazole, and complicated urinary tract infections, including pyelonephritis in patients with limited alternative treatment options.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
