Procedural Sedation and Analgesia in the Emergency Department
June 1, 2019
Reprints
AUTHORS
Caleb P. Canders, MD, Department of Emergency Medicine, UCLA Ronald Reagan Medical Center, Los Angeles
Gregory E. Tong, MD, Department of Emergency Medicine, UCLA Ronald Reagan Medical Center, Los Angeles
Nadine El Fawal, University of California at Los Angeles
PEER REVIEWER
Frank LoVecchio, DO, FACEP, Vice-Chair for Research, Medical Director, Samaritan Regional Poison Control Center, Emergency Medicine Department, Maricopa Medical Center, Phoenix, AZ
EXECUTIVE SUMMARY
- The Centers for Medicare and Medicaid Services (CMS) acknowledges the unique capabilities of emergency physicians to perform procedural sedation and analgesia. CMS permits hospitals to follow specialty guidelines such as those produced by the American College of Emergency Physicians (ACEP) in determining hospital policy.
- ACEP’s recent guideline on unscheduled sedation supports the use of ketamine and propofol as appropriate sedation agents when used by qualified emergency physicians.
- Fasting should not be required for sedation. End-tidal CO2 monitoring does not appear to reduce adverse events.
- Sedative agents should be selected based on the condition of the patient and the type of procedure to be performed.
Introduction
Procedural sedation and analgesia (PSA), commonly referred to as “conscious sedation” or “procedural sedation,” is performed in the emergency department (ED) to alleviate anxiety, decrease pain, and provide amnesia to patients undergoing painful procedures or diagnostic imaging.1,2 Occasionally, there is a need to achieve deep sedation in the ED, in which a patient is unarousable but will respond purposefully following repeated or painful stimuli. In contrast, general anesthesia is a state of unconsciousness and apnea.3 The goal of PSA is to achieve moderate sedation, where the patient has depressed consciousness but responds to verbal commands with or without tactile stimulation, or dissociation, where the patient enters a trance-like state. The ideal agent for PSA in the ED provides anxiolysis, analgesia, and amnesia in a rapid and predictable manner, without side effects, and with a quick recovery phase.4 This paper will review guidelines for performing PSA in the ED, including suggested training, preprocedural assessment, and intraprocedural monitoring. In addition, it will describe some of the commonly used medications for PSA in the ED.
Guidelines for ED PSA
Physicians from multiple specialties routinely perform PSA in various inpatient and outpatient settings, such as the ED, the endoscopy suite, the operating room, and the intensive care unit. As a result, different medical societies have developed unique, specialty-specific guidelines about how PSA should be performed and monitored. A single hospital may have multiple guidelines governing how PSA is performed in different settings. In 2001, the Joint Commission on Accreditation of Health Care Organizations (now known as The Joint Commission) created a policy statement that defined the spectrum of PSA and created qualitative measurements for providers based on many of the standards set forth by the American Society of Anesthesiologists (ASA).5 However, most ASA guidelines are based on evidence from scheduled, elective PSA encounters, whereas PSA performed in the ED generally is unplanned and emergent. As a result, the American College of Emergency Physicians (ACEP) developed and updated its own PSA guidelines.6 This practice has been endorsed by the Centers for Medicare and Medicaid Services (CMS), which states: “The ED is a unique environment where patients present on an unscheduled basis with often very complex problems that may require several emergent or urgent interventions to proceed simultaneously to prevent further morbidity and mortality.”7 The authors of this paper agree that hospital-based guidelines on PSA performed in the ED and privileging should focus on whether the emergency provider possesses the knowledge and experience in airway assessment and management, and the rescue skills that might be needed during PSA performed in emergent situations.
PSA Training, Privileging, and Quality Assurance
PSA, advanced airway management (e.g., intubation), vascular access, and resuscitation are considered core competencies in emergency medicine residency training. Emergency medicine residents are expected to have performed at least 15 supervised PSA encounters by graduation.8 As a result, most emergency medicine-trained physicians are proficient in PSA. For other providers involved in emergent PSA, ACEP’s guidelines support what are known as “focused PSA privileges,” in which providers demonstrate knowledge and skills pertinent to moderate sedation and do not intend to perform dissociative or deep sedation, although they still possess rescue skills since patients may pass unintentionally to a deeper level of sedation.6 Often, department medical directors and hospital PSA committees specify “focused PSA privileges” based on an individual evaluation of each provider’s competency. PSA curricula, consisting of self-learning and simulation sessions, have been shown to improve provider proficiency in performing PSA, independent of provider profession and clinical experience.9 Despite wide variability in the credentialing processes, training, and equipment used in different EDs, studies have shown overall low rates of adverse events for PSA in the ED, regardless of the level of training of the provider.10 Departmental and institutional quality assurance (QA) programs should track adverse events, review documentation, and identify areas for improvement. Although CMS suggests a single physician oversee sedation in a hospital, that physician does not need to be an anesthesiologist.11
Personnel for Emergent PSA
ACEP guidelines recommend having at least two trained healthcare providers present during PSA, with at least one who is skilled in vascular access.6 ACEP, ASA, and The Joint Commission guidelines agree that the provider who oversees the PSA, usually a physician, should be responsible for the patient’s global management, including supervision of personnel and identification and management of adverse events.6,12 In a “single-coverage” ED, this provider also may be expected to perform other brief procedures (e.g., fracture/dislocation reduction), as long as the non-PSA procedure can be halted.13-15 In ACEP’s guidelines, the second provider, usually a nurse or respiratory therapist, primarily is involved with continuous monitoring of the patient and documentation, although this provider can assist with minor, interruptible tasks that do not interfere with monitoring.6 In practice, often a respiratory therapist and one to two nurses are present and share the duties of monitoring and documentation. In addition, another provider may be available to help with non-PSA procedures. Preprocedural preparation and intraprocedural distraction by child life specialists also have been shown to decrease pain and distress in children undergoing procedures.16
Nurse-Administered PSA Medications
Qualified nurses routinely administer sedating medications and paralytics for non-PSA indications, such as intubation, under the supervision of an ordering provider. However, without supporting evidence, many state and nursing regulatory bodies restrict nurses from administering PSA medications in the ED. There is ample evidence that it is safe for nurses to administer PSA medications with the ordering provider present and specifying the medication type and dose.6,17-19
Pre-PSA Assessment
When possible, a pre-sedation assessment of a patient should include a focused history and physical exam, including a review of comorbidities, medications, allergies, and prior PSA and anesthesia experiences. Anatomic variants that may put a patient at risk of airway or ventilatory compromise, such as obesity and obstructive airway disease, should be noted. The ASA physical status classification system is used commonly to identify patients at risk for adverse events.20 (See Table 1.) Patients at the extremes of age and with severe systemic disease (i.e., ASA III-VI) have a greater risk of adverse events during PSA.4,21,22 Of note, the Mallampati score, which is a visual assessment of the oropharynx, has been shown to be unreliably assessed between providers, poorly predicts difficult bag-valve mask ventilation and intubation, and has no impact on clinical outcomes. Therefore, it cannot be recommended as a necessary part of the pre-PSA evaluation.23-27
Table 1. American Society of Anesthesiologists (ASA) Classification |
||
|
ASA Classification |
Description |
Example |
|
ASA I |
Normal healthy patient |
No medical problems and no/minimal smoking |
|
ASA II |
Patient with mild systemic disease |
Smoking, pregnancy, obesity, well-controlled diabetes or hypertension, mild lung disease |
|
ASA III |
Patient with severe systemic disease that is not incapacitating |
Poorly controlled diabetes or hypertension, chronic obstructive pulmonary disease, end-stage renal disease, congestive heart failure with ejection fraction 25-40%, history of transient ischemic attack (TIA) or coronary artery disease |
|
ASA IV |
Life-threatening, severe systemic disease |
Recent history of myocardial infarction or stroke/ TIA, ongoing cardiac ischemia or severe valve dysfunction, implanted implantable cardioverter-defibrillator, ejection fraction < 25% |
|
ASA V |
Moribund patient not expected to survive without procedure |
Ruptured abdominal or thoracic aortic aneurysm, intracranial bleed with mass effect, ischemic bowel |
|
ASA VI |
Brain dead patient undergoing organ harvesting |
|
|
Data from: Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class) In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441940/. Accessed May 14, 2019. |
||
Preprocedural Fasting
Based on the traditional thinking that fasting time contributes to aspiration risk, many PSA guidelines include requirements for preprocedural fasting. The ASA consensus statement recommends that patients not eat solid foods for six hours or drink clear liquids for two hours prior to PSA or general anesthesia.28 However, these requirements may not be practical for ED patients. In addition, given that airway reflexes are left intact, there should be no increase in the risk of aspiration.29 The authors of one study in children undergoing PSA found that although a majority did not meet fasting criteria, there were no aspiration episodes.30 In addition, there have been no reports in the literature of deaths from aspiration during ED PSA.3,31 As a result, ACEP guidelines do not consider recent food intake to be a contraindication to PSA, although it may affect the timing and target level of sedation.1,32,33 In patients with underlying risk factors for aspiration, such as small bowel obstruction, a PSA medication that preserves protective airway reflexes, such as ketamine, is preferred.34
Monitoring and Equipment
It is standard practice and consistent with ACEP guidelines to continuously monitor the patient’s pulse, oxygen saturation, and respiratory rate during PSA in the ED. Blood pressure typically is measured every five minutes.6 Clinically significant arrhythmias or hypotensive episodes are extremely rare during PSA in individuals without systemic disease. Supplemental oxygen, suction, and monitoring equipment should be available. In addition, advanced airway equipment, resuscitative medications, and vascular access supplies should be readily accessible.
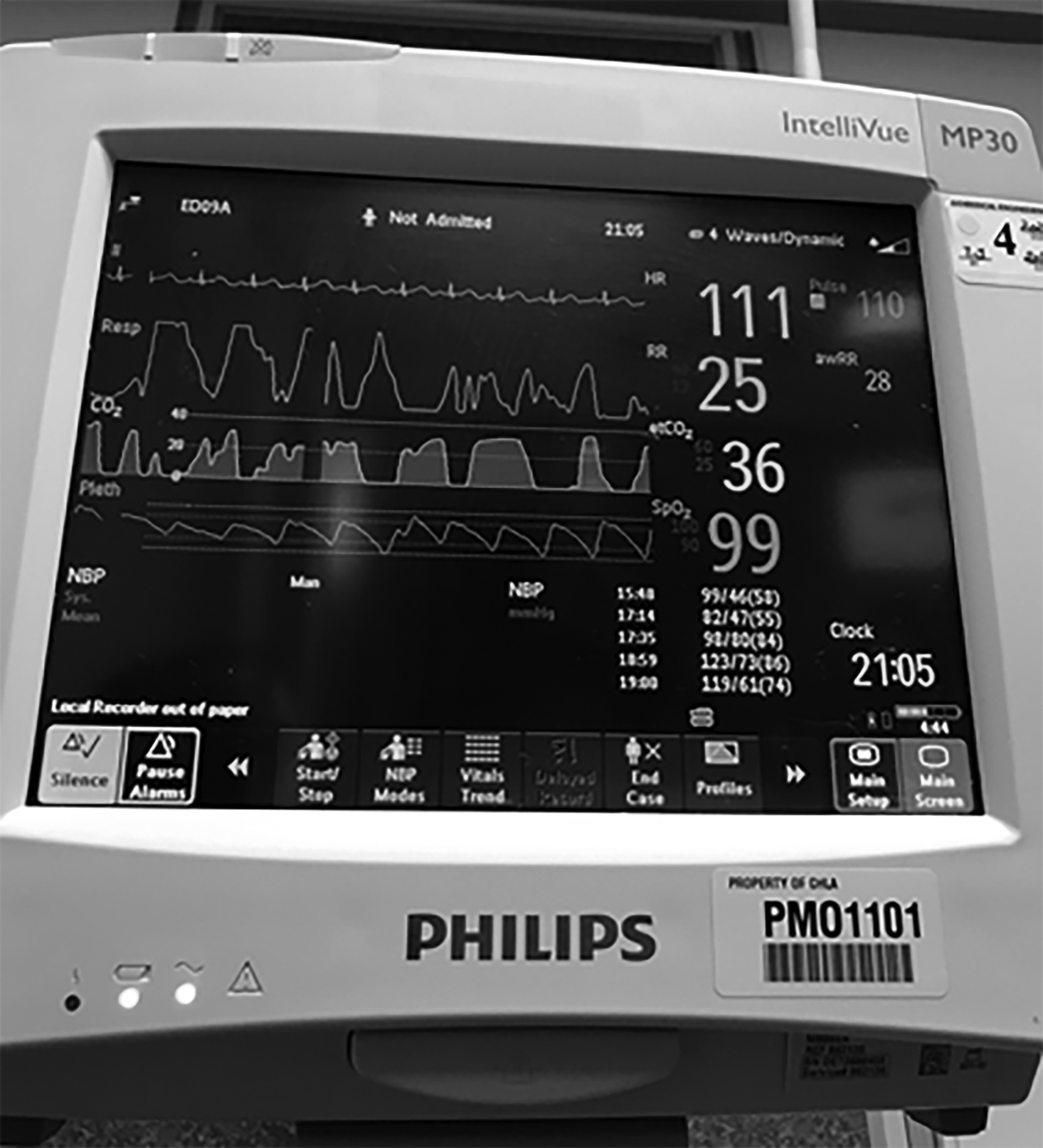
Carbon Dioxide (ETCO2) Monitoring
End-tidal carbon dioxide (ETCO2) monitoring may be used to detect early signs of respiratory depression.3,35 (See Figure 1.) Supplemental oxygen also can be administered during PSA without affecting ETCO2 readings. However, ETCO2 monitoring during PSA in the ED is expensive and has not been shown to predict episodes of desaturation or reduce the frequency of clinically significant adverse events.36-40 In addition, transient decreased ventilation or apnea detected with ETCO2 may lead to an unnecessary intervention, such as positive pressure ventilation, which can cause gastric insufflation and an increased risk of aspiration.41 Therefore, ETCO2 monitoring is not yet considered standard of care in ED PSA.
Figure 1. Monitoring |
 |
Monitoring of Responsiveness
Responsiveness may serve as an indirect indicator of patient distress, eventual procedural recall, and ventilatory adequacy.6 Several sedation/responsiveness scales may be used during PSA, although none have been found to be superior in the evaluation of sedation.42 Examples include the Observer’s Assessment of Alertness/Sedation (OAA/S) Scale, the Ramsay Sedation Scale, and the Richmond Agitation-Sedation Scale.43,44 In the 2018 update of the ACEP practice guidelines, the authors argue that patient responsiveness to voice or pain at any given moment is functionally irrelevant, as long as the patient has effective ventilation.6
Discharge Criteria
Following PSA, the provider should document the PSA course, including drugs and doses administered, adverse events, and rescue interventions. Patients typically are monitored until they return to baseline vital signs, level of consciousness, and neuromuscular activity.45 Per ACEP guidelines, it is not necessary to demonstrate that a patient is able to tolerate an oral challenge after PSA, since nausea is common and usually self-resolving.6 Serious adverse effects rarely occur after 30 minutes from final medication administration.46
Adverse Events
Adverse events are defined as unexpected and undesirable responses to medications that threaten to cause or cause injury or discomfort to a patient.11,47 Serious adverse events associated with ED PSA are extremely rare and have a reported incidence of < 1% in most studies.1,48 Apnea (usually defined as cessation of breathing > 30 seconds), with or without oxygen desaturation, is the most commonly reported adverse event and occurs in approximately 40 per 1,000 sedations.12,49 However, many apneic episodes resolve with tactile or verbal stimulation of the patient and have no clinical consequences. A review of PSA performed in 3,636 patients in the ED found that only two underwent unplanned intubations. Other adverse events associated with PSA vary according to the medication used and the patient population, and may include laryngospasm, bronchospasm, cardiovascular instability, paradoxical reactions, emergence reactions, and emesis.48 (See Table 2.) Clinically significant hemodynamic changes are rare in patients without serious cardiovascular disease.6 Delayed side effects, such as restlessness and imbalance, have been reported in children who have received PSA.50 Adverse events associated with specific PSA medications will be discussed later.
Table 2. Adverse Events Associated With Specific Procedural Sedation and Analgesia Medications |
|
|
Medication |
Associated Adverse Events |
|
Propofol |
Respiratory depression, hypotension, decreased cardiac output |
|
Etomidate |
Nausea, vomiting, myoclonus, adrenal suppression |
|
Ketamine |
Tachycardia, hypertension, increased cardiac output, hypersalivation, emergence phenomenon, laryngospasm |
|
Midazolam |
Hypoventilation , hypoxemia, hypotension, paradoxical stimulatory effect |
|
Fentanyl |
Pruritus, nausea, vomiting, respiratory depression |
Pediatric PSA
Most of the medications used for PSA in adults that are reviewed in the following sections also are safe in children. About 60% of pediatric PSA performed outside the operating room is related to obtaining radiologic imaging and, therefore, does not require analgesia.51 In addition, although rare, adverse events are more common in very young children, those with underlying disease, and when multiple sedatives are coadministered.52
Commonly Used PSA Medications
The choice of PSA medications should be customized to the patient and procedure being performed. Almost all of the medications used to perform PSA in the ED are considered off-label, despite being safe when administered by providers with proper training.53 There is inadequate evidence to guide PSA medication selection in pregnancy, although, in most instances, the exposures to medications are brief, the doses are low, and significant adverse pregnancy outcomes are not expected.6,54 In general, elderly patients require lower initial loading doses of PSA medications than younger adults (commonly half the dose) because of decreased clearance, and medications are titrated to effect. (See Table 3.)
Table 3. Some Medications for Procedural Sedation and Analgesia in the Emergency Department34,57,85,102,116,120,147,148 |
||||
|
Medication |
Typical Initial Dosing |
Repeat Dosing if Needed |
Onset |
Duration |
|
Propofol |
IV 0.5 mg/kg over 3 to 5 minutes |
IV 0.5 mg/kg every 3 to 5 minutes |
30 seconds |
3 to 10 minutes |
|
Etomidate |
IV 0.1 to 0.2 mg/kg push over 30 seconds |
IV 0.1 mg/kg every 3 to 5 minutes |
< 1 minute |
5 to 10 minutes |
|
Ketamine |
IV 1 to 2 mg/kg over 30 to 60 seconds |
IV 0.5 to 1 mg/kg every 5 to 15 minutes |
< 30 seconds |
5 to 10 minutes |
|
IM 4 to 5 mg/kg |
IM 2 to 5 mg/kg after 5 to 10 minutes |
3 to 4 minutes |
12 to 25 minutes |
|
|
Midazolam |
IV 0.5 to 2 mg over 2 minutes |
Repeat every 2 to 3 minutes |
1 to 3 minutes |
30 to 60 minutes |
|
IN (peds) 0.2 to 0.5 mg/kg given 15 minutes prior to procedure |
Within 5 minutes |
20 minutes |
||
|
Fentanyl |
IV 0.5 to 1 mcg/kg |
Repeat every 2 minutes, maximum total dose of 5 mcg/kg |
< 1 minute |
30 to 60 minutes |
|
IV = intravenous; IM = intramuscular; IN = intranasal |
||||
Propofol
Propofol is a sedative-hypnotic medication with amnestic and sedative properties, but without analgesic properties.55,56 It is the most commonly used agent for PSA, with rapid onset (less than one minute), short duration (less than 10 minutes), and predictable, dose-dependent potency. Propofol typically is given as an initial intravenous dose of 0.5 to 1 mg/kg over three to five minutes, followed by repeat intravenous boluses of 0.5 mg/kg every three to five minutes as needed for adequate sedation.57 Studies have shown that the recovery time from propofol is shorter than from midazolam or ketamine, and patients report higher satisfaction with propofol than with benzodiazepines.58-60 Propofol also has been shown to have antioxidant properties and to decrease the cerebral metabolic rate, which is protective in the setting of brain injury.61-63 Metabolism of propofol is not affected by liver or kidney disease.64 Elderly patients typically require lower doses than younger patients because of decreased clearance.64,65 Propofol can cause dose-related respiratory depression, hypotension, and decreased cardiac output; however, this rarely leads to unplanned intubation, prolonged observation, or complications requiring admission.65-67 It is often combined with the analgesic fentanyl, which may increase the rates and severity of adverse events. Hypotension associated with propofol may be mitigated when it is combined with ketamine, which tends to increase blood pressure.68 (See the section on “ketofol.”) Nausea and vomiting are rare side effects of propofol.
In children, propofol is given as a loading bolus of 1 to 2 mg/kg intravenously, followed by 0.5 to 1 mg/kg boluses until adequate sedation is achieved.69-71 Children who receive propofol have faster recovery than those who receive midazolam.58 Desaturations are reported in up to 13% of children, but most resolve spontaneously. Only 3% of children require airway maneuvers and less than 1% require bag-valve mask ventilation.70-72 Up to 92% of children who receive propofol have transient decreases in systolic blood pressure, typically without clinical signs of decreased perfusion.72
Lipid Emulsion. Propofol is prepared in a lipid solvent that induces bradykinin release at the site of injection. As a result, patients who receive propofol via a peripheral vein commonly report pain at the injection site.73,74 Lidocaine (commonly 1 mL of the 1% concentration) can be given intravenously just prior to or coadministered with propofol to prevent this effect.75 Short-term infusions of propofol have not been shown to affect serum lipid levels.64 Allergies to soybeans or eggs are contraindications to propofol use.76 The lipid emulsion also is a potential medium for bacterial contamination after being opened, punctured, and stored at a room temperature for a prolonged period of time.77
Etomidate
Etomidate is an imidazole sedative-hypnotic.55 It has no analgesic properties and often is coadministered with fentanyl.78 Given its rapid onset (five to 15 seconds), short duration (five to 10 minutes), and rapid recovery time, it is especially useful for short procedures, such as joint dislocation reduction or obtaining some imaging in children.79-82 In addition, etomidate maintains hemodynamic stability in the setting of cardiovascular compromise, making it an ideal agent for cardioversion or PSA in hypotensive patients.83,84 Typically, it is given as an intravenous bolus of 0.1 to 0.2 mg/kg over 30-60 seconds. Repeat boluses of the same dose can be given every three to five minutes as needed.85 Common adverse events include nausea and vomiting, which may be resistant to antiemetics, and myoclonus, typically characterized by brief tremors.86,87 In rare cases of severe myoclonus or myoclonus interfering with procedures, treatment consists of midazolam
1 to 2 mg intravenous given every 60 seconds until the myoclonus resolves.85,88 Respiratory depression leading to oxygen desaturation of < 90% or apnea occurs in only 10% of patients.87
Reversal and dose-dependent adrenal suppression have been reported after even a single dose of etomidate.55,89,90 As a result, the use of a single dose of etomidate for PSA in septic patients has been debated.91,92 The hypnotic and adrenocortical suppressive effects of etomidate also are prolonged in patients with liver disease.84
Ketamine
Ketamine, a synthetic derivative of phencyclidine, induces a dissociation between the thalamocortical and limbic systems of the brain, producing a trance-like state that prevents perception of or response to painful stimuli. It provides amnesia, sedation, and analgesia.93 Ketamine preserves airway reflexes, spontaneous breathing, and cardiac output, making it a popular choice for PSA in the ED. It has been shown to have a success rate greater than 90% in ED PSA, as defined by high patient satisfaction, adequate analgesia, and lack of procedural recall.94 An added benefit of ketamine is that it may prevent central sensitization to pain and secondary hyperalgesia to episodes of acute pain.95-97 When given intravenously, typically as a dose of 1 to 2 mg/kg over 30-60 seconds, ketamine produces clinical effects that occur within one minute and last for approximately 15 minutes.34,65,97 When given intramuscularly, typically as a dose of 4 to 5 mg/kg, its onset is delayed by five minutes and the effects can be more prolonged and variable.34 Ketamine also can be given via intranasal, intrarectal, and intrathecal routes.98 Patients who receive ketamine typically return to mental baseline within 30-60 minutes after administration. The concurrent use of benzodiazepines or barbiturates may decrease recovery agitation but also can prolong the effects of ketamine. Elderly patients have lower clearance and prolonged duration of action of ketamine compared to younger patients.99
Ketamine is the most commonly used agent for PSA in children undergoing painful procedures in the ED and receives ACEP’s highest level of recommendation based on available evidence.100 It is not recommended for use in children younger than 3 months of age because of concerns for laryngospasm, airway obstruction, and apnea.34 Pediatric dosing via the intravenous and intramuscular routes is the same as the adult dosing.
Because of its inhibition of catecholamine reuptake, ketamine can cause sympathomimetic effects, such as tachycardia, hypertension, and increased cardiac output. Caution should be used in patients with known or suspected coronary artery disease.98 The theory that ketamine increases intracranial pressure has been debunked, and the medication has been shown to be safe in patients with traumatic brain injury.101 Laryngospasm, thought to be due to a heightened gag reflex, occurs in less than 2% of patients, is usually transient, and often responds to brief bag-valve mask ventilation.65 The increased secretions associated with ketamine can be pretreated with glycopyrrolate (typically 0.2 mg intravenous) or atropine (typically 0.01 mg/kg intravenous, maximum dose 0.5 mg). Respiratory depression associated with ketamine is rare but has been reported with rapid intravenous administration of ketamine in neonates and in patients with central nervous system injuries.102 Nausea and vomiting, which develop in approximately 4% of patients who receive ketamine, typically occur once the patient is alert and have not been associated with increased rates of aspiration.103 These symptoms may be prevented with administration of ondansetron.
“Emergence phenomenon,” characterized by agitation or hallucinations during recovery from ketamine, occur in approximately 50% of adults and 10% of children who receive the medication.104 Administering a small, concurrent dose of midazolam (e.g., 0.05 mg/kg intravenous) decreases the incidence of emergence phenomenon.105,106 Ketamine should be avoided in patients with schizophrenia since it may exacerbate psychotic symptoms.
Ketofol
Ketofol is the term coined for the combination of ketamine and propofol, which can be mixed in advance or dosed sequentially. The two medications are thought to have complementary and synergistic effects. Propofol may lessen the nausea associated with ketamine.98 In addition, the sympathomimetic effects of ketamine may reduce the hypotension associated with propofol.107 Ketofol has been shown to be safer and similarly effective when compared with fentanyl in adults undergoing ED PSA.108 In a study of pediatric PSA comparing intravenous ketofol (0.5 mg/kg of each component) to intravenous ketamine alone (1 mg/kg), researchers found fewer episodes of nausea and vomiting in the ketofol group, no significant differences in sedation or recovery times, and no clinically significant adverse events in any of the patients.109 Physicians and nurses have reported higher satisfaction when using ketofol.110-113
Midazolam
Midazolam is a benzodiazepine with both amnestic and anxiolytic properties. Given its lack of analgesic properties, it often is combined with an opiate during ED PSA. It is the preferred benzodiazepine for PSA because of its rapid onset (one to three minutes when given intravenously), short duration (30-60 minutes), superior amnestic properties, and low risk of phlebitis.3,114-116 Typically, it is given as an intravenous bolus of
0.5 to 2 mg over two minutes. Its effects may be prolonged in patients who are obese or elderly or who have renal or kidney disease.66,116,117 Midazolam also can be given via oral, intramuscular, rectal, intranasal, and intraosseous routes.65 When given orally, higher doses usually are required, given its high first-pass metabolism, and its effects are less predictable.116 When given intramuscularly, there is usually slow and unpredictable absorption.118,119 Intranasal midazolam (commonly given as a 0.2 to 0.5 mg/kg dose), administered via a mucosal atomizer, provides approximately 20 minutes of sedation and is useful for obtaining diagnostic imaging in children or performing minor procedures, such as simple laceration repair.120-122 It is not licensed for use in children younger than 6 months of age.69
Adverse events associated with midazolam include hypoventilation, hypoxemia, and hypotension, which typically are seen with high doses of midazolam. Administration of repeated doses can lead to prolonged sedation due to the accumulation of midazolam and its metabolites.116,123 When midazolam is combined with opiates, there is a synergic effect on sedation and respiratory depression, which can lead to apnea.124 Hiccups may occur after administration of midazolam and usually resolve spontaneously.65 Medications that inhibit the liver metabolic enzyme p450 oxidase, such as cimetidine, hasten the onset of midazolam and may lead to oversedation.65 Midazolam crosses the placenta and is category D in pregnancy. A paradoxical, stimulatory effect can occur in some patients who receive midazolam.125
Flumazenil, the reversal agent for midazolam and other benzodiazepines, is administered to overly sedated patients who have received benzodiazepines. Typically, it is given as an intravenous dose of 0.2 mg, repeated every one minute for a maximum dose of 3 mg. In children, an intravenous dose of 0.01 mg/kg is given. The use of flumazenil is controversial in ED PSA, since it can precipitate seizures and subsequent airway compromise in patients with a tolerance to benzodiazepines.126 Flumazenil-precipitated seizures are refractory to benzodiazepines and usually are treated with phenobarbital or propofol.
Fentanyl
Fentanyl is a synthetic opioid with mainly analgesic properties often used in conjunction with other PSA medications. It lacks amnestic or sedative properties. It is the preferred opioid for PSA because of its rapid onset (less than one minute when given intravenously), short duration of action (30-60 minutes), and minimal cardiovascular effects.127,128 Its high lipid-solubility causes it to be 100 times more potent than morphine in equivalent doses.129 Because of its unpredictable absorption via other routes, it usually is given intravenously for PSA. It typically is administered as an intravenous dose of 0.5 to 1 mcg/kg every two minutes until desired analgesia is achieved, with a maximum total dose of 5 mcg/kg.3 Fentanyl is used commonly in combination with midazolam when other short-acting PSA agents are unavailable. In such cases, smaller doses of fentanyl (e.g., 0.5 mcg/kg intravenous) should be given over longer intervals to minimize the synergistic effects of the medications and subsequent oversedation.
Fentanyl is eliminated primarily by the liver and should be used with caution in patients with liver disease. Infants have a slower metabolism of fentanyl and should receive approximately one-third the normal dose.65 Common adverse events associated with fentanyl include pruritus, nausea, and vomiting. Fentanyl also decreases responsiveness to the stimulatory effects of carbon dioxide, leading to a dose-dependent depression in ventilation.65 High doses have been reported to cause chest wall and glottic rigidity, leading to difficulty with ventilation, although this has not been reported during ED PSA.130
Naloxone acts as an antagonist at opioid receptors and is the reversal agent for fentanyl and other opioids. It is administered as a 0.02 to 0.20 mg intravenous dose, given every two to three minutes until adequate ventilation is achieved while maintaining pain control.131 In children, a 0.001 to 0.005 mg/kg intravenous dose is administered.132
Other PSA Medications
Dexmedetomidine
Dexmedetomidine is a highly selective alpha2-receptor agonist with sedative, anxiolytic, and analgesic properties that has been used for deep sedation. It is a relatively novel medication for ED PSA. It has a rapid onset of action (10-14 minutes), quick recovery time, and high success rate in PSA.69,133,134 Dexmedetomidine should be used with caution in patients with hemodynamic instability. It also has the ability to potentiate opiates and other sedating medications. Dexmedetomidine has been shown to be effective in pediatric PSA to obtain radiologic imaging and in patients undergoing dental procedures.135-138 In adults, it typically is given as a 1 mcg/kg loading dose over 10 minutes, followed by a maintenance infusion of 0.6 mcg/kg per hour.139 In children, it typically is given as 0.5 to 3 mcg/kg intravenous boluses, followed by a maintenance infusion of 0.5 to 2 mcg/kg/hour.129,135,137,140 Dexmedetomidine also can be given by the intranasal route.141 The adverse effects of dexmedetomidine (e.g., bradycardia, hypotension) counteract the adverse effects of ketamine (e.g., tachycardia, hypertension), making the combination of the two agents a potentially useful regimen for ED PSA.142 Dexmedetomidine is eliminated mainly by the liver, and dosing should be reduced in patients with liver disease.139
Barbiturates
Methohexital, a barbiturate with a rapid onset of action and duration of less than 10 minutes, has been used for ED PSA for short procedures, such as reductions and cardioversion. It produces a state of unconsciousness but does not provide analgesia. It directly depresses myocardial and respiratory ventilatory centers, which can cause hypotension and apnea, limiting its use in certain populations.65 Pentobarbital, another barbiturate with a rapid onset of action that can be given via oral, intramuscular, or intravenous routes, has been used to obtain pediatric diagnostic imaging. However, this drug has a long recovery time, limiting its use in the ED.143-145
Chloral Hydrate
Chloral hydrate, a sedative-hypnotic, can be given orally or rectally, which makes it appealing for use in children. However, its onset of action can be delayed up to 60 minutes and its effects can last for hours, making it less useful for PSA performed in the ED.65,143 In addition, a high incidence of adverse events (notably vomiting) and a high failure rate limit its use.144,146
Inhaled Agents
Nitrous oxide, an inhaled gas with ultrashort-acting (three to five minutes) sedative, anxiolytic, and analgesic properties, has been used in the past for PSA in the ED. However, its risks of malignant hyperthermia and hypotension, its potential for misuse, and its equipment requirements (e.g., a well-ventilated room with a scavenger system) limit its use.3
Conclusion
PSA is a core competency of emergency medicine. Guidelines for performing PSA in the ED vary by institution, but usually are set by hospital-based committees and are based on standards set by medical societies, such as ACEP. Preprocedural fasting is no longer considered to be a requisite in performing PSA in the ED. ETCO2 monitoring has not been shown to reduce rates of adverse events and is not considered standard of care.
There is no single ideal drug for PSA, and emergency providers should understand which medications are best suited for certain patients undergoing certain procedures, as well at the adverse events associated with each medication. In a healthy patient without comorbidities, propofol often is preferred, given its rapid onset and quick recovery. For short procedures and in patients with cardiovascular instability, etomidate is ideal. Ketamine is useful in patients with cardiovascular compromise, given its catecholaminergic effects and limited effects on respiratory drive, and is highly effective in children. Intranasal midazolam may be useful in pediatric procedures in which minimal sedation is required and intravenous access is unnecessary, such as diagnostic imaging. Newer agents, such as dexmedetomidine, may be used more commonly for PSA in the future. Serious adverse events, although extremely rare, should be anticipated in all episodes of PSA. Emergency providers who are trained in airway management, vascular access, and resuscitation are uniquely qualified to address such events and to apply rescue maneuvers.
REFERENCES
- American College of Emergency Physicians. Clinical policy for procedural sedation and analgesia in the emergency department. Ann Emerg Med 1998;31:663-677.
- Green SM, Krauss B. Procedural sedation terminology: Moving beyond “conscious sedation.” Ann Emerg Med 2002;39:433-435.
- Bahn EL, Holt KR. Procedural sedation and analgesia: A review and new concepts. Emerg Med Clin North Am 2005;23:503-517.
- Gan TJ. Pharmacokinetic and pharmacodynamic characteristics of medications used for moderate sedation. Clin Pharmacokinet 2006;45:855-869.
- Joint Commission on Accreditation of Healthcare Organization. 2001 sedation and anesthesia care standards. Oakbrook Terrace, IL: JCAHO.
- American College of Emergency Physicians. Unscheduled Procedural Sedation: A Multidisciplinary Consensus Practice Guideline. Approved by the ACEP Board, Sept. 28, 2018. Available at: https://www.acep.org/patient-care/policy-statements/unscheduled-procedural-sedation-a-multidisciplinary-consensus-practice-guideline/. Accessed May 10, 2019.
- Centers for Medicare & Medicaid Services. State Operations Manual. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Accessed April 24, 2019.
- ACGME Emergency Medicine Defined Key Index Procedure Minimums Review Committee for Emergency Medicine 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramResources/EM_Key_Index_Procedure_Minimums_103117.pdf. Accessed April 1, 2019.
- Sauter T, et al. Interprofessional and interdisciplinary simulation-based training leads to safe sedation procedures in the emergency department. Scand J Trauma Resusc Emerg Med 2016;24:97.
- Shavit I, et al. Sedation provider practice variation: A survey analysis of pediatric emergency subspecialists and fellows. Pediatr Emerg Care 2010;26:742-747.
- Roback MG, et al. Tracking and Reporting Outcomes of Procedural Sedation (TROOPS): Standardized quality improvement and research tools from the International Committee for the Advancement of Procedural Sedation. Br J Anaesth 2018;120:164-172.
- Chawla N, et al. Procedural sedation in the ICU and emergency department. Curr Opin Anaesthesiol 2017;30:507-512.
- O’Connor RE, et al. American College of Emergency Physicians. Procedural sedation and analgesia in the emergency department: Recommendations for physician credentialing, privileging, and practice. Ann Emerg Med 2011;58:365-370.
- Josephy CP, Vinson DR. Feasibility of single- vs two-physician procedural sedation in a small community emergency department. Am J Emerg Med 2018;36:977-982.
- Sacchetti A, et al. Procedural sedation in the community emergency department: Initial results of the ProSCED registry. Acad Emerg Med 2007;14:41-46.
- Stephens BK, et al. Techniques to comfort children during stressful procedures. Accid Emerg Nurs 1999;7:226-236.
- Walker JA, et al. Nurse-administered propofol sedation without anesthesia specialists in 9152 endoscopic cases in an ambulatory surgery center. Am J Gastroenterol 2003;98:1744-1750.
- Tohda G, et al. Propofol sedation during endoscopic procedures: Safe and effective administration by registered nurses supervised by endoscopists. Endoscopy 2006;38:360-367.
- Rex DK, et al. Trained registered nurses/endoscopy teams can administer propofol safely for endoscopy. Gastroenterology 2005;129:1384-1391.
- Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441940/. Accessed May 10, 2019.
- Caperell K, Pitetti R. Is higher ASA class associated with an increased incidence of adverse events during procedural sedation in a pediatric emergency department? Pediatr Emerg Care 2009;25:661-664.
- Miner JR, et al. Procedural sedation of critically ill patients in the emergency department. Acad Emerg Med 2005;12:124-128.
- Lee A, et al. A systematic review (meta-analysis) of the accuracy of the Mallampati tests to predict the difficult airway. Anesth Analg 2006;102:1867-1878.
- Baker P. Assessment before airway management. Anesthesiol Clin 2015;33:257-278.
- Karkouti K, et al. Inter-observer reliability of ten tests used for predicting difficult tracheal intubation. Can J Anaesth 1996;43:554-559.
- Iyer MS, et al. Higher Mallampati scores are not associated with more adverse events during pediatric procedural sedation and analgesia. West J Emerg Med 2018;19:430-436.
- Ezri T, et al. The incidence of class “zero” airway and the impact of Mallampati score, age, sex, and body mass index on prediction of laryngoscopy grade. Anesth Analg 2001;93:1073-1075.
- [No authors listed]. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: A report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999;90:896-905.
- Thorpe RJ, Benger J. Preprocedural fasting in emergency sedation. Emerg Med J 2010;27:254-261.
- Agrawal D, et al. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med 2003;42:636-645.
- Bhatt M, et al. Association of preprocedural fasting with outcomes of emergency department sedation in children. JAMA Pediatr 2018;172:678-685.
- Godwin SA, et al. Clinical policy: Procedural sedation and analgesia in the emergency department. Ann Emerg Med 2005;45:177-196.
- Green SM. Fasting is a consideration — not a necessity — for emergency department procedural sedation and analgesia. Ann Emerg Med 2003;42:647-650.
- Green SM, et al. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med 2011;57:449-461.
- Green SM, Pershad J. Should capnographic monitoring be standard practice during emergency department procedural sedation and analgesia? Pro and con. Ann Emerg Med 2010;55:265-267.
- Dewdney C, et al. Capnography for procedural sedation in the ED: A systematic review. Emerg Med J 2017;34:476-484.
- Mohr NM, et al. Using continuous quantitative capnography for emergency department procedural sedation: A systematic review and cost-effectiveness analysis. Intern Emerg Med 2018;13:75-85.
- Deitch K, et al. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med 2010;55:258-266.
- Sivilotti ML, et al. A comparative evaluation of capnometry versus pulse oximetry during procedural sedation and analgesia on room air. CJEM 2010;12:397-404.
- Wall BF, et al. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database Syst Rev 2017;3:CD010698.
- Smally AJ, et al. Procedural sedation and analgesia in the emergency department. Curr Opin Crit Care 2011;4:317-322.
- Williams MR, et al. Efficacy outcome measures for procedural sedation clinical trials in adults: An ACTTION systematic review. Anesth Analg 2016;122:152-170.
- Chernik DA, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J Clin Psychopharmacol 1990;10:244-251.
- Frenzel D, et al. Is the bispectral index appropriate for monitoring the sedation level of mechanically ventilated surgical ICU patients? Intensive Care Med 2002;28:178-183.
- Joint Commission on Accreditation of Healthcare Organizations. Care of patients: Examples of compliance. In: Joint Commission on Accreditation of Healthcare Organizations, Oakbrook Terrace, IL; 1999:87-91.
- Newman DH, et al. When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1367 pediatric procedural sedations. Ann Emerg Med 2003;42:627.
- Mason KP, et al; International Sedation Task Force. Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: A consensus document from the World SIVA International Sedation Task Force. Br J Anaesth 2012;108:13-20.
- Miller MA, et al. Procedural sedation and analgesia in the emergency department: What are the risks? Emerg Med Clin North Am 2005;23:551-572.
- Bellolio MF, et al. Incidence of adverse events in adults undergoing procedural sedation in the emergency department: A systematic review and meta-analysis. Acad Emerg Med 2016;23:119-134.
- Malviya S, et al. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics 2000;105:E42.
- Cravero JP, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: Report from the Pediatric Sedation Research Consortium. Pediatrics 2006;118:1087-1096.
- Leroy PL, et al. Professional skills and competence for safe and effective procedural sedation in children: Recommendations based on a systematic review of the literature. Int J Pediatr 2010;2010:934298.
- Green SM, Krauss B. Barriers to propofol use in emergency medicine. Ann Emerg Med 2008;52:392-398.
- Neuman G, Koren G. Safety of procedural sedation in pregnancy. J Obstet Gynaecol Can 2013;35:168-173.
- Brohan J, Goudra BG. The role of GABA receptor agonists in anesthesia and sedation. CNS Drugs 2017;31:845-856.
- Sanna E, et al. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: Actions of the anesthetics propofol and pentobarbital. Mol Pharmacol 1995;47:213-217.
- Miner JR, Burton JH. Clinical practice advisory: Emergency department procedural sedation with propofol. Ann Emerg Med 2007;50:182.
- Havel CJ Jr, et al. A clinical trial of propofol vs. midazolam for procedural sedation in a pediatric emergency department. Acad Emerg Med 1999;6:989-997.
- Vardi A, et al. Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Crit Care Med 2002;30:1231-1235.
- Lameijer H, et al. Propofol versus midazolam for procedural sedation in the emergency department: A study on efficacy and safety. Am J Emerg Med 2017;35:692-696.
- Ansley DM, et al. Propofol cardioprotection for on-pump aortocoronary bypass surgery in patients with type 2 diabetes mellitus (PRO-TECT II): A phase 2 randomized-controlled trial. Can J Anesth 2016;63:442-453.
- De Cosmo G, et al. Changes in hemodynamics during isoflurane and propofol anesthesia: A comparative study. Neurol Res 2005;27:433-435.
- Johnston AJ, et al. Effects of propofol on cerebral oxygenation and metabolism after head injury. Br J Anaesth 2003;91:781-786.
- Fulton B, Sorkin EM. Propofol. An overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs 1995;50:636-657.
- Blackburn P, Vissers R. Pharmacology of emergency department pain management and conscious sedation. Emerg Med Clin North Am 2000;18:803-827.
- Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology 2005;103:20-24.
- Burton JH, et al. Propofol for emergency department procedural sedation and analgesia: A tale of three centers. Acad Emerg Med 2006;13:24-30.
- Weaver CS, et al. Emergency department procedural sedation with propofol: Is it safe? J Emerg Med 2007;33:355-361.
- Starkey E, Sammons HM. Sedation for radiological imaging. Arch Dis Child Educ Pract Ed 2011;96:101-106.
- Dalal PG, et al. Sedation and anesthesia protocols used for magnetic resonance imaging studies in infants: Provider and pharmacologic considerations. Anesth Analg 2006;103:863-868.
- Pershad J, et al. Comparison of propofol with pentobarbital/midazolam/fentanyl sedation for magnetic resonance imaging of the brain in children. Pediatrics 2007;120:e629-e636.
- Bassett KE, et al. Propofol for procedural sedation in children in the emergency department. Ann Emerg Med 2003;42:773-782.
- Grauers A, et al. Propofol infusion rate does not affect local pain on injection. Acta Anaesthesiol Scand 2002;46:361-363.
- Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth 1999;83:397-404.
- Scott RP, et al. Propofol: Clinical strategies for preventing the pain of injection. Anaesthesia 1988;43:492-494.
- Hofer KN, et al. Possible anaphylaxis after propofol in a child with food allergy. Ann Pharmacother 2003;37:398-401.
- Wachowski I, et al. The growth of microorganisms in propofol and mixtures of propofol and lidocaine. Anesth Analg 1999;88:209-212.
- Brown TB, et al. Procedural sedation in the acute care setting. Am Fam Physician 2005;71:85.
- Vinson DR, Bradbury DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med 2002;39:592-598.
- Burton JH, et al. Etomidate and midazolam for reduction of anterior shoulder dislocation: A randomized, controlled trial. Ann Emerg Med 2002;40:496-504.
- Baxter AL, et al. Etomidate versus pentobarbital for computed tomography sedations: Report from the Pediatric Sedation Research Consortium. Pediatr Emerg Care 2007;23:690-695.
- Kienstra AJ, et al. Etomidate versus pentobarbital for sedation of children for head and neck CT imaging. Pediatr Emerg Care 2004;20:499-506.
- Uchida I, et al. Etomidate potentiation of GABAA receptor gated current depends on the subunit composition. Neurosci Lett 1995;185:203-206.
- Erdoes G, et al. Etomidate – a review of robust evidence for it use in various clinical scenarios. Acta Anaesthesiol Scand 2014;58:380-389.
- Miner JR, et al. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med 2007;49:15.
- Ruth WJ, et al. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med 2001;8:13-18.
- Falk J, Zed PJ. Etomidate for procedural sedation in the emergency department. Ann Pharmacother 2004;38:1272-1277.
- McDowall RH, et al. Total intravenous anesthesia for children undergoing brief diagnostic or therapeutic procedures. J Clin Anesth 1995;7:273-280.
- Keim SM, et al. Etomidate for procedural sedation in the emergency department. Pharmacotherapy 2002;22:586-591.
- Allolio B, et al. Adrenocortical suppression by a single induction dose of etomidate. Klin Wochenschr 1984;62:1014-1017.
- Jackson WL Jr. Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock? A critical appraisal. Chest 2005;127:1031-1038.
- Walls RM, Murphy MF. Clinical controversies: Etomidate as an induction agent for endotracheal intubation in patients with sepsis: Continue to use etomidate for intubation of patients with septic shock. Ann Emerg Med 2008;52:13-14.
- Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013;19:370-380.
- Sih K, et al. Ketamine in adult emergency medicine: Controversies and recent advances. Ann Pharmacother 2011;45:1525-1534.
- McGuinness SK, et al. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med 2011;12:1551-1558.
- Laskowski K, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911-923.
- Chaparro LE, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307.
- Parashchanka A, et al. Role of novel drugs in sedation outside the operating room: Dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol 2014;27:442-447.
- Tsui BC, et al. Regional anaesthesia in the elderly: A clinical guide. Drugs Aging 2004;21:895-910.
- Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med 2004;44:460-471.
- Zeiler FA, et al. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care 2014;21:163-173.
- Rosenbaum SB, Palacios JL. Ketamine. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019.
- Taylor PA, Towey RM. Depression of laryngeal reflexes during ketamine anaesthesia. Br Med J 1971;2:688-689.
- Sacchetti A, et al. Pediatric analgesia and sedation. Ann Emerg Med 1994;23:237-250.
- Chudnofsky CR, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med 2000;7:228-235.
- Sener S, et al. Ketamine with and without midazolam for emergency department sedation in adults: A randomized controlled trial. Ann Emerg Med 2011;57:109-114.e2.
- Alletag MJ, et al. Ketamine, propofol, and ketofol use for pediatric sedation. Pediatr Emerg Care 2012;28:1391-1395.
- Messenger DW, et al. Subdissociative-dose ketamine versus fentanyl for analgesia during propofol procedural sedation: A randomized clinical trial. Acad Emerg Med 2008;15:877-886.
- Shah A, et al. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med 2011;57:425-433.e2.
- David H, Shipp J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med 2011;57:435.
- Phillips W, et al. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: Clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother 2010;24:349.
- Miner JR, et al. Randomized, double-blinded, clinical trial of propofol, 1:1 propofol/ketamine, and 4:1 propofol/ketamine for deep procedural sedation in the emergency department. Ann Emerg Med 2015;65:479.
- Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 2007;49:23-30.
- Coughlin MW, Panuska HJ. Direct comparison of midazolam and diazepam for conscious sedation in outpatient oral surgery. Anesth Progr 1989;36:160-163.
- Wright SW, et al. Comparison of midazolam and diazepam for conscious sedation in the emergency department. Ann Emerg Med 1993;22:201-205.
- Horn E, Nesbit SA. Pharmacology and pharmacokinetics of sedatives and analgesics. Gastrointest Endosc Clin N Am 2004;14:247-268.
- MacGilchrist AJ, et al. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut 1986;27:190-195.
- Hubbard AM, et al. Sedation for pediatric patients undergoing CT and MRI. J Comput Assist Tomogr 1992;16:3-6.
- Ramoska EA, et al. Midazolam use in the emergency department. J Emerg Med 1991;9:247-251.
- Chiaretti A, et al. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child 2011;96:160-163.
- Harcke HT, et al. Sedation in pediatric imaging using intranasal midazolam. Pediatr Radiol 1995;25:341-343.
- Klein EJ, et al. A randomized clinical trial comparing oral, aerosolized intranasal, and aerosolized buccal midazolam. Ann Emerg Med 2011;58:323-329.
- Bauer TM, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995;346:145-147.
- Bailey PL, et al. Respiratory effects of midazolam and fentanyl: Potent interaction producing hypoxemia and apnea. Anesthesiology 1988;69:A813.
- Massanari M, et al. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr (Phila) 1997;36:681-684.
- Seger DL. Flumazenil — treatment or toxin. J Toxicol Clin Toxicol 2004;42:209.
- Jagoda AS, et al. Clinical policy for procedural sedation and analgesia in the emergency department. Ann Emerg Med 1998;31:663-677.
- Borland ML, et al. Intranasal fentanyl reduces acute pain in children in the emergency department: A safety and efficacy study. Emerg Med (Fremantle) 2002;14:275-280.
- Chudnofsky CR, et al. The safety of fentanyl use in the emergency department. Ann Emerg Med 1989;18:635-639.
- Streisand JB, et al. Fentanyl-induced rigidity and unconsciousness in human volunteers. Incidence, duration, and plasma concentrations. Anesthesiology 1993;78:629-634.
- Lavonas EJ, et al. Part 10: Special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(Suppl 2):S501-S518.
- Hegenbarth MA; American Academy of Pediatrics Committee on Drugs. Preparing for pediatric emergencies: Drugs to consider. Pediatrics 2008;121:433-443.
- Candiotti KA, et al. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg 2010;110:47-56.
- Bergese SD, et al. A Phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther 2010;17:586-595.
- Heard CM, et al. Dexmedetomidine for pediatric MRI sedation: A review of a series of cases. Paediatr Anaesth 2007;17:888-892.
- Mason KP, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth 2008;18:403-411.
- Mason KP, et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg 2006;103:57-62.
- Ogawa S, et al. Intravenous sedation with low-dose dexmedetomidine: Its potential for use in dentistry. Anesth Prog 2008;55:82-88.
- Weerink MAS, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017;56:893-913.
- McMorrow SP, Abramo TJ. Dexmedetomidine sedation: Uses in pediatric and procedural sedation outside the operating room. Pediatr Emerg Care 2012;28:292-296.
- Gyanesh P, et al. Comparison between intranasal dexmedetomidine and intranasal ketamine as premedication for procedural sedation in children undergoing MRI: A double-blind, randomized, placebo-controlled trial. J Anesth 2014;28:12-18.
- Tobias JD. Dexmedetomidine and ketamine: An effective alternative for procedural sedation? Pediatr Crit Care Med 2012;13:423-427.
- Pereira JK, et al. Comparison of sedation regimens for pediatric outpatient CT. Pediatr Radiol 1993;23:341-344.
- Mason KP, et al. Infant sedation for MR imaging and CT: Oral versus intravenous pentobarbital. Radiology 2004;233:723-728.
- Moro-Sutherland DM, et al. Comparison of intravenous midazolam with pentobarbital for sedation for head computed tomography imaging. Acad Emerg Med 2000;7:1370-1375.
- Malviya S, et al. Pentobarbital vs chloral hydrate for sedation of children undergoing MRI: Efficacy and recovery characteristics. Paediatr Anaesth 2004;14:589-595.
- Blayney MR. Procedural sedation for adult patients: An overview. Continuing Education in Anaesthesia Critical Care & Pain 2012;12:176-180.
- Bahn EL, Holt KR. Procedural sedation and analgesia: A review and new concepts. Emerg Med Clin North Am 2005;23:503.
Procedural sedation and analgesia (PSA) is performed in the emergency department (ED) to alleviate anxiety, decrease pain, and provide amnesia to patients undergoing painful procedures or diagnostic imaging.This article will review guidelines for performing PSA in the ED, including suggested training, preprocedural assessment, and intraprocedural monitoring.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
