Diagnosis and Management of Infants With Critical Congenital Heart Disease in the Emergency Department
December 1, 2019
Reprints
Related Articles
-
Walking at Work: A Helping Hand for a Healthier Heart
-
Examining the Effects of Cannabis on the Heart
-
Lifestyle Interventions and HbA1c in Prediabetic Patients
-
Rapid Reversal of Anticoagulation Reduces Mortality from Intracerebral Hemorrhage
-
Outcomes Are Better for Acute Stroke Patients Who Arrive Rapidly at Endovascular-Capable Centers
AUTHORS
Jamie Colombo, DO, Assistant Professor of Pediatric Cardiology, University of Arizona, Tucson
Preston J. Boyer, MD, Pediatric Resident, University of Arizona, Tucson
Shelby White, MD, FACC, Assistant Professor of Pediatric Cardiology, University of Virginia, Charlottesville
PEER REVIEWER
Larry B. Mellick, MD, MS, FAAP, FACEP, Vice Chairman for Academic Affairs, Interim Section Chief of Pediatric Emergency Medicine, Assistant Residency Director, Professor of Emergency Medicine, University of South Alabama, Mobile
EXECUTIVE SUMMARY
• Critical congential heart disease (CCHD) refers to heart defects present at birth that, when left untreated, can present emergently within the first year of life with a significant risk of mortality without prompt intervention. Two-thirds of these infants will die in the first year without appropriate intervention.
• The three most common presentations of CCHD are important to keep in mind: shock, cyanosis, and respiratory distress.
• Shock in the infant can manifest early, with poor feeding and fussiness, and progress to lethargy. The hallmark of shock is hypoperfusion, often presenting with poor capillary refill and weak pulses. Cardiogenic shock also can have nonspecific signs, such as tachycardia, cyanosis, restlessness, oliguria, bradycardia, or altered mental status, and may overlap with presentations of heart failure with diaphoresis, edema, and hepatomegaly.
• Cyanosis related to congential heart disease (CHD) is central in nature, which typically can be observed in the mucous membranes or the trunk. The differential diagnosis for cyanosis in the infant includes persistent pulmonary hypertension, CHD, and primary lung disease; however, cyanosis without respiratory distress is almost always associated with structural CHD and should prompt a diagnostic workup for cardiac disease. Hypoxia due to lung disease should improve with the administration of oxygen, whereas hypoxia that remains despite the use of 100% oxygen could indicate CHD.
• Respiratory distress as a result of CHD can be a manifestation of cardiogenic shock in neonates or due to overcirculation related to a large left-to-right shunt lesion. Overcirculation typically presents after 6 weeks of age and is a result of excessive pulmonary blood flow.
• Infants with coarctation of the aorta typically present in the first two to six weeks of life, and more commonly between days three through seven of life, when the ductus arteriosus begins to close, resulting in the development of aortic arch obstruction and reduced systemic perfusion. As a result, patients can begin to manifest signs of hypoperfusion to the lower body, diaphoresis with feeds, tachypnea, retractions, and cyanosis. Infants who present acutely during this period can have concurrent signs of renal failure, such as oliguria or anuria, mesenteric ischemia, and metabolic acidosis. Common physical findings are a gallop and absent, delayed, or weak pulses in the lower extremities. A high blood pressure gradient between upper and lower extremities (> 20 mmHg) can be present. Also there can be a disparity between upper and lower extremity pulse oximetry readings.
• Tetralogy of Fallot represents between 3 and 10% of all CHD. Classically, it has been defined by four distinct anomalies: ventricular septal defect, overriding aorta, right ventricular outflow tract obstruction, and right ventricular hypertrophy. Infants with the most severe right ventricular outflow tract obstruction are dependent on the ductus arteriosus for pulmonary blood flow. They present with profound cyanosis during the first weeks of life as the ductus begins to close. Clubbing, dyspnea, or hypoxic spells also can be present in an older infant or child.
• An infant with tetralogy of Fallot may present with periods of intense fussiness and cyanosis, which are typical of hypoxic spells (“tet spell”). These are demonstrated by rapid and deep respirations, irritability and prolonged crying, cyanosis (increased from baseline), and decreased intensity of the murmur. Severe spells can lead to seizures, flaccid tone, stroke, or death. Treatment starts with calming the child by relieving pain or anxiety. Increase systemic vascular resistance and reduce the right-to-left shunting by holding the infant in a knee-chest position to increase the intraabdominal pressure. Morphine, which can be administered intramuscularly or subcutaneously, and midazolam, which can be given intranasally, can help the child remain calm and help suppress the respiratory center to reverse the right-to-left shunting. Volume can be infused to optimize preload. In severe hypoxic spells with acidosis, treatment with sodium bicarbonate, ketamine, or propranolol also may be helpful.
• Early initiation of prostaglandin E1 (PGE) is important in the course of CCHD or tetralogy of Fallot with severe pulmonary stenosis or pulmonary atresia because many CHD cases that present in the first two to four weeks of life are dependent on the ductus arteriosus. The most common risks of PGE include apnea and hypotension, so the infant should be monitored closely to observe for any hypercapnia or hypoxia.
Background
Critical congenital heart disease (CCHD) is a significant cause of morbidity and mortality in children. When children with undiagnosed congenital heart disease (CHD) present acutely, the challenge of diagnosis and the importance of timely management can be daunting for any physician in an emergency setting. The children with the highest morbidity and mortality from critical congenital heart disease are infants younger than 1 year of age.
Despite advances in screening for congenital heart disease, some children leave the newborn nursery with undiagnosed congenital heart disease, and present acutely to the emergency department. The three main categories of presentation are cyanosis, respiratory distress, and shock. While history and physical exam can be helpful, it is important to have a low threshold to consider congenital heart disease and initiate a workup for cardiac disease in children who present with any of these signs or symptoms. Increased familiarity with the presentations, underlying pathophysiology, and acute management of critical congenital heart disease will enhance awareness of subtle presentations and critical aspects of treatment.
Emergency physicians should work closely with pediatric cardiologists when congenital heart disease is suspected and use tools such as prostaglandin E1, which is often the most time-sensitive and important intervention for these infants.
Introduction
For the emergency provider, congenital heart disease in infants can present a significant diagnostic challenge. Presenting signs and symptoms often are not specific for cardiac disease, and they can overlap with presentations of sepsis, respiratory disease, or metabolic abnormalities. This review focuses on the evaluation and assessment of critically ill infants to recognize CCHD in these patients. CCHD refers to heart defects present at birth that, when left untreated, can present emergently within the first year of life with a significant risk of mortality without prompt intervention. These infants almost always need rapid diagnosis and treatment, so emergency providers should be familiar with these presentations.
Epidemiology
CHD represents the spectrum of heart defects present at birth and is the most common congenital defect, with an incidence of about 1% of all newborns.1 Between 25% and 30% of children with CHD will have conditions severe enough to require invasive treatment within the first year of life, which defines their heart defects as “critical.”2 Two-thirds of those infants will die in the first year without appropriate intervention.3 Death from CHD is the most common cause of mortality related to a congenital anomaly in all infants < 1 year of age, and the mortality rate for infants < 1 year of age with CHD is 35 times higher than the overall mortality rate of CHD. Each year, more than 50% of deaths attributed to CHD are in infants < 1 year of age.4,5 Infants who have a delayed diagnosis of critical CHD until after hospital discharge have a mortality rate of up to 30%.6 These data emphasize that children < 1 year of age are at the greatest risk of death from unrecognized CHD.7
Early identification of CHD is the goal of pediatricians and pediatric cardiologists. Prenatal ultrasound screening has been used since the late 1980s, and more recently, universal pulse oximetry screening at 24 hours of life has been implemented widely. Despite these tools, accurate and timely diagnosis of all patients with CHD remains limited. During physical examination of the newborn, the gold standard in neonatal screening, subtle findings can be missed, such as decreased lower extremity pulses. In addition, many symptoms, such as a patent ductus arteriosus, cutis marmorata, or acrocyanosis, can be masked by normal newborn findings. Prenatal ultrasound is estimated to detect 25% of CHD, and prior to the widespread implementation of in-hospital pulse oximetry screening, it was estimated that 25-50% of infants with CHD were discharged from the hospital without diagnosis.8-13 In 2009, the American Academy of Pediatrics recommended universal pulse oximetry screening for asymptomatic neonates before hospital discharge.10 Then, in 2011, the Department of Health and Human Services formally added the CHD pulse oximetry screen, or CCHD screening, to the Recommended Uniform Screening Panel. Nearly all states have adopted some form of legislation to implement the recommended screening, but there is a wide variation in the procedural technique, timing after birth, and specifications of the screening across states.14 Even at centers where universal CCHD pulse oximetry screening has been implemented, it is estimated to have a sensitivity of about 70%, with a wide variation between studies evaluating its efficacy (0-100%).10 However, even with the success of current newborn screening practices, 13-20% of patients with CCHD and up to 50% of those with noncritical CHD can be discharged from the newborn nursery undiagnosed.6,15 For the emergency physician, it is important to be aware of these limitations and to know that infants with CCHD still can present emergently with an undiagnosed cardiac defect.2 Infants who are not identified by routine screenings are at the highest risk for morbidity and mortality related to their heart disease; thus, preparation for the rapid identification and management of these patients is crucial.3,12
Physiology
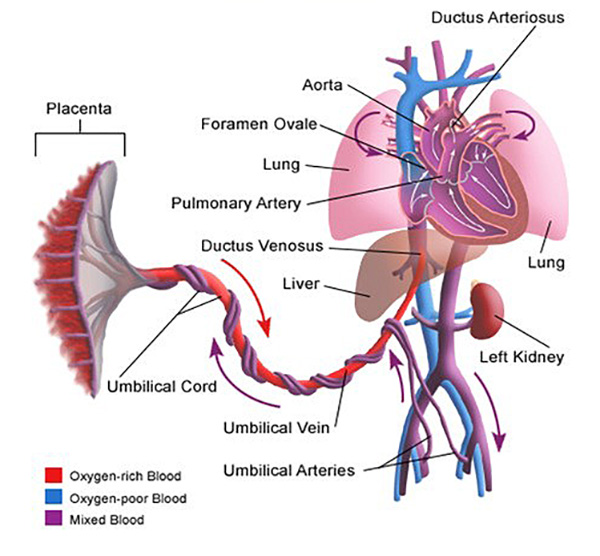
An understanding of fetal and transitional postnatal cardiovascular physiology is important for quick recognition of the infant with a cardiac anomaly. In utero, blood oxygenation is dependent on the placenta. Fetal blood flows through the umbilical arteries to the low-pressure system of the placenta, where it is oxygenated in parallel with maternal blood. Oxygenated blood returns through the umbilical vein back to the central venous system via the ductus venosus in the liver. As oxygenated blood enters the right atrium through the inferior vena cava, most of it is directed through the foramen ovale directly into the left atrium. The oxygenated blood that does pass into the right ventricle is pumped against the relatively high-pressure system of the lungs. Because of this, most of the oxygenated blood pumped from the right ventricle passes from the pulmonary arteries through the ductus arteriosus into the aorta for systemic distribution. These natural mechanisms bypass the separate right and left circulations, which allow the fetus to survive in utero, even with a significant congenital heart lesion.16 (See Figure 1.)
Figure 1. Diagram of Normal Fetal Circulation |
 |
|
Reprinted from Paediatrics and Child Health, Vol. 28, Issue 12, Lawford A, Tulloh R MR, "Cardiovascular adaptation to extra uterine life," 549-555, 2018, with permission from Elsevier. |
As infants are born, the low pressure sink of the placenta is reversed immediately when the umbilical cord is clamped. This increases the pressure in the systemic arterial system, and thereby the left side of the heart. Simultaneously, as oxygen enters the lungs for the first time, vasodilation begins to occur in the pulmonary vascular bed, reducing the resistance of the pulmonary vasculature. During the first hours to days of life, the venous blood returning from the body is now directed to the lower pressure system of the lungs to receive oxygen. Over the next few days, the ductus arteriosus closes, mediated by factors such as oxygen.3,17
In the normal newborn, this transition creates two separate pathways of blood flow from the heart: one to the lungs, and the other to the body. In the infant with obstructive CCHD (see “Obstructive Lesions”), at least one of these pathways is unable to function on its own, and either the lungs or the body is deprived of blood flow unless it can be shunted from the other pathway. Flow is maintained through a patent ductus arteriosus, atrial septal defect, ventricular septal defect, or a combination of these. If these shunts are unable to share sufficient flow between both pathways (such as when the ductus arteriosus begins to close), the infant can progress quickly to heart failure, shock, arrest, and death. This often happens in the first few days to weeks of life.
For infants with left-to-right shunt lesions (see “Left-to-Right Lesions”), typically both pathways are intact, but there remains an abnormal connection between the two. Over time, because of lower resistance in the lungs, too much blood is directed into the pulmonary pathway, creating respiratory distress, pulmonary edema, and heart failure.
Understanding the time frame for the transition of circulation gives context to the presentations of CHD and can help identify undiagnosed infants in an acute setting.3,16
The Challenge of Diagnosis
The presentation of an infant or child with undiagnosed cardiac disease in the emergency department can present a significant challenge to the emergency provider.2,18,19 While cardiac disease, specifically structural cardiac defects, can have a wide range of clinical presentation,20 it is important to keep cardiac disease as a part of the differential diagnosis when infants or children present with findings of respiratory distress, cyanosis, or shock.3,21 Specific aspects of the patient history, clinical presentation, physical exam, and initial workup can help identify infants at risk of cardiac conditions and encourage specific diagnostic tests earlier in the emergency department course.
Clinical Presentation. The clinical presentation of CCHD is broad and can range from a child in extremis to mimicking a simple childhood condition. The three most common presentations of CCHD are important to keep in mind: shock, cyanosis, and respiratory distress.3
Shock in the infant can manifest early, with poor feeding and fussiness, and progress to lethargy. The hallmark of shock is hypoperfusion, often presenting with poor capillary refill and weak pulses. Cardiogenic shock also can have nonspecific signs, such as tachycardia, cyanosis, restlessness, oliguria, bradycardia, or altered mental status, and may overlap with presentations of heart failure with diaphoresis, edema, and hepatomegaly.22
Cyanosis related to CHD is central in nature, which typically can be observed in the mucous membranes or the trunk. The differential diagnosis for cyanosis in the infant includes persistent pulmonary hypertension, CHD, and primary lung disease; however, cyanosis without respiratory distress is almost always associated with structural CHD and should prompt a diagnostic workup for cardiac disease.3 Hypoxia due to lung disease should improve with the administration of oxygen, whereas hypoxia that remains despite the use of 100% oxygen could indicate CHD.
Respiratory distress as a result of CHD can be a manifestation of cardiogenic shock in neonates or due to overcirculation related to a large left-to-right shunt lesion. Overcirculation typically presents after 6 weeks of age and is a result of excessive pulmonary blood flow. While the timing of symptoms can be insidious, patients with respiratory distress from CHD can deteriorate rapidly over the course of hours to days. With large left-to-right shunts, early signs of respiratory distress are feeding difficulties, diaphoresis, irritability, and tachypnea that result in poor weight gain and failure to thrive.
History
A thorough history includes evaluation of prenatal course, peripartum events, and postpartum care, as well as family history. Maternal prenatal risk factors for CHD include smoking in the first trimester, exposure to secondhand smoke, obesity, diabetes mellitus or gestational diabetes, preeclampsia, folate deficiency, infection (specifically rubella or chlamydia), binge drinking, and certain medications.2 (See Table 1.) Twins, specifically monozygotic twins, are at higher risk of CHD, and half of all patients with trisomy 21 have CHD.23 Other genetic syndromes, such as DiGeorge syndrome, Williams syndrome, and Turner syndrome, also have an increased risk of CHD.4,24 (See Table 2.)
Table 1. Proposed Association of Congenital Heart Disease With Particular Drugs/Exposures |
|
|
Drug/Exposure |
Proposed Associated Cardiac Lesion |
|
Paroxetine |
ASD, VSD, ventricular outflow tract obstruction |
|
Buproprion |
Left ventricular outflow tract obstruction |
|
Valproic acid |
ASD, VSD, tetralogy of Fallot |
|
Nitrofurantoin |
Hypoplastic left heart, ASD |
|
Cephalosporins |
ASD |
|
Lithium |
Ebstein’s anomaly, mitral atresia |
|
Ibuprofen |
Transposition of the great vessels, VSD |
|
Vitamin A |
Pulmonary stenosis, outflow tract abnormalities |
|
ASD: Atrial septal defect; VSD: Ventricular septal defect Adapted from Lynch TA, Abel DE. Teratogens and congenital heart disease. J Diagn Med Sonogr 2015;31:301-305. |
|
Table 2. Most Common Congenital Heart Defects Associated With Genetic Disorders |
|
|
Down syndrome (trisomy 21) |
Endocardial cushion defects (atrioventricular canal, atrial septal defect, ventricular septal defect) |
|
Turner syndrome (monosomy X) |
Bicuspid aortic valve, coarctation of the aorta |
|
Williams syndrome |
Supravalvar aortic stenosis |
|
DiGeorge syndrome |
Conotruncal anomalies (tetralogy of Fallot, truncus arteriosus, interrupted aortic arch) |
Critical Congential Heart Lesions
The following is a brief review of the critical congenital heart lesions encountered most often. These reviews emphasize the emergent presentations of these conditions in the newborn and infant periods.
Left-to-Right Lesions
Case Presentation. An 8-week-old infant girl is transferred to the emergency department via ambulance from a rural hospital several hours away. The call from emergency medical services reports that she is being transferred for respiratory distress. Despite having an oxygen saturation of 98%, she was placed on 1-2 L/min of 100% FiO2 via nasal cannula for tachypnea.
On arrival, the infant is noted to have a rectal temperature of 37.2° C. Blood glucose is 70, and the oxygen saturation is 100% on 2 L/min of oxygen via nasal cannula. Heart rate is 150 beats per minute (bpm). Respiratory rate is 80 breaths per minute. Blood pressure is normal. The infant is nontoxic appearing, although breathing rapidly. She is awake and alert, with some generalized hypotonia, and normal infantile reflexes intact. Her facial characteristics are consistent with trisomy 21. The fontanelle is soft and flat, the oral mucosa is pink, and the palate is intact. Cardiac exam reveals a mildly tachycardic rate, regular rhythm, with a low, rumbling diastolic murmur heard at the apex. Lungs have mild fine crackles bilaterally, with adequate aeration. She has no abnormalities of the extremities, and no rashes or skin lesions.
The infant was born at 37 and 2/7 weeks gestational age, and the mother reports that she has had a normal newborn course until about a week ago. Since that time, she has started to take longer amounts of time to feed and has been drinking less volume than before. She seems to have to stop frequently during feeds to breathe hard and often will be very sweaty over her head and face during feeds.
An echocardiogram is performed, which reveals a large ventricular septal defect.
Ventricular Septal Defects. Ventricular septal defects are the most common form of CHD.25 They account for up to 37% of all CHD, not including those associated with a cyanotic heart lesion. The hemodynamic significance of a ventricular septal defect is determined by the amount of shunting that occurs, which is directly related to the size of the defect and the pulmonary vascular resistance to systemic vascular resistance ratio. Large defects typically present around 6-8 weeks of life, as this is when the pulmonary vascular resistance drops to its nadir.25
Prior to the reduction of pulmonary vascular resistance, ventricular septal defect shunting will be insignificant; however, as pulmonary vascular resistance drops, left-to-right shunting will increase through the ventricular septal defect. Large ventricular septal defects will result in increased pulmonary blood flow and volume load on the left ventricle. (See Figure 2.) Infants present with tachypnea, intolerance of oral feedings, and failure to thrive. Small ventricular septal defects can present with a holosystolic murmur in the absence of symptoms, but large ventricular septal defects may not have an associated murmur until the shunt becomes quite significant. A chest X-ray may show pulmonary congestion and an enlarged cardiac silhouette. An electrocardiogram may show left and right ventricular hypertrophy but typically is unremarkable in an infant.
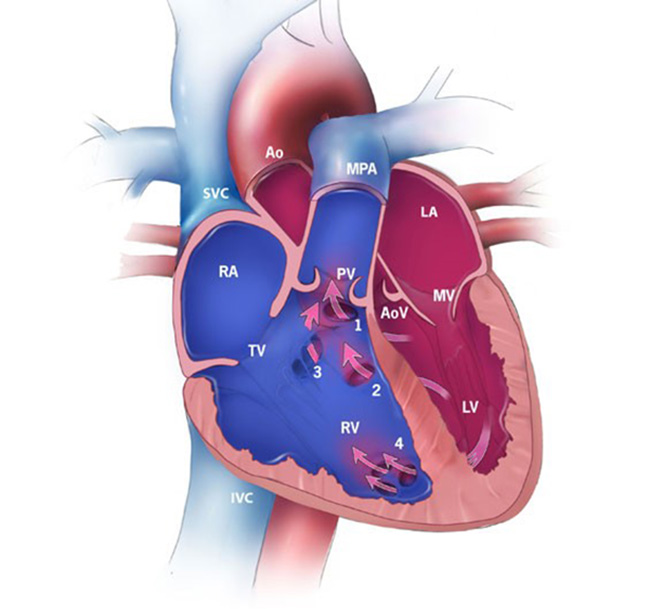
Figure 2. Ventricular Septal Defect |
 |
|
RA: Right atrium; RV: right ventricle; LA: left atrium; LV: left ventricle; SVC: superior vena cava; IVC: inferior vena cava; MPA: main pulmonary artery; Ao: aorta; TV: tricuspid valve; MV: mitral valve; PV: pulmonary valve; AoV: aortic valve; 1: conoventricular, malaligned; 2: perimembranous; 3: inlet; 4: muscular. |
Symptoms may be controlled with diuretics, but definitive management of large defects requires surgical closure.
Patent Ductus Arteriosus. In utero, the ductus arteriosus is vital, redirecting blood from the pulmonary artery to the aorta. After a term infant is born, the ductus typically will close within the first 72 hours of life, mitigated by a number of factors, including oxygen concentration and circulating prostaglandins. Delay or failure of the ductus to close results in an increasing left-to-right shunt as the pulmonary vascular resistance drops.16
In term infants, patent ductus arteriosus accounts for 5-10% of all CHD. In addition, patent ductus arteriosus affects premature infants disproportionately; it is estimated that up to 80% of infants weighing < 1,000 g at birth have a patent ductus arteriosus.26
Infants with a large patent ductus arteriosus have unrestricted flow from left to right and a large volume burden on the pulmonary vascular bed. A neonatal patent ductus arteriosus will have a cross-sectional area roughly the same size as the descending aorta. In addition, there will be some component of vascular “steal,” which results from the partial runoff of cardiac output into the patent ductus arteriosus during both systole and diastole. This affects downstream perfusion of peripheral organs. Partly because of this vascular steal, patent ductus arteriosus in premature infants has been linked to necrotizing enterocolitis, spontaneous bowel perforation, neurodevelopmental delay, intraventricular hemorrhage, respiratory distress syndrome, and bronchopulmonary dysplasia. The result is a clinical presentation like that of a large ventricular septal defect. Infants will present with symptoms related to pulmonary overcirculation, as discussed previously.27
A continuous or a systolic murmur is typically auscultated. Pulse pressure can be wide, evidenced by bounding distal pulses. Chest X-rays will demonstrate cardiomegaly from left atrial and left ventricular enlargement, with increased pulmonary vascular markings. (See Figure 3.) An electrocardiogram may demonstrate left ventricular or biventricular hypertrophy.
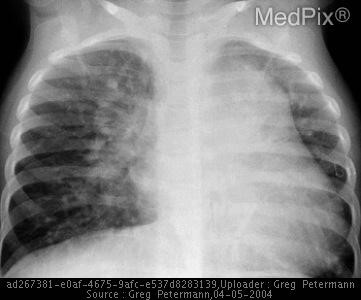
Figure 3. Patent Ductus Arteriosus |
 |
|
Pulmonary congestion and large cardiac silhouette from an enlarged left atrium in an infant with a patent ductus arteriosus. Reprinted from MedPix. https://medpix.nlm.nih.gov/topic?id=b4a5d497-61b5-49ac-bf56-99700142de56. |
Initial stabilization includes diuresis, and definitive management can include either surgical or percutaneous closure.
Obstructive Lesions
Case Presentation. An 8-day-old infant is brought to the emergency department for one day of fast breathing and fussiness. His mother reports that she noticed his breathing becoming more rapid the night before presentation. He has not been feeding well and only had two or three wet diapers in the past 24 hours. His temperature taken at home was 35.8° C. His mother denies rhinorrhea or congestion.
He was born at 40 weeks to a gravida 3, para 3 mother. There were no complications during pregnancy. Prenatal ultrasounds were normal. The birth was complicated by Group B Streptococcus-positive mother, who received inadequate antibiotic treatment less than four hours prior to delivery.
On evaluation, the infant is tachypneic, with coarse breath sounds, having marked subcostal and sternal retractions, with a respiratory rate ranging from 75-100 breaths per minute. Heart rate is 193 bpm. Blood pressure is unable to be read with multiple attempts on the lower extremities. He has mottling diffusely, but it is worse in the lower extremities. Otherwise his initial evaluation and physical exam are unremarkable. Temperature on arrival is 35.8, but one hour later the temperature is 34.9. Blood cultures, complete blood count, metabolic panel, urinalysis, and urine culture are ordered, but attempts at blood draws are unsuccessful. After several attempts, a femoral vein puncture is performed to collect blood for these studies. A lumbar puncture is performed, and cerebral spinal fluid is collected. Treatment with antibiotics and acyclovir is initiated. Rapid viral screens are obtained and results are negative.
While awaiting laboratory results, the patient continues to have increased work of breathing and decreased oxygen saturation. He is placed on oxygen support via nasal cannula. Venous blood gas shows a pH of 6.8, pCO2 of 73, lactate of 3.5, and bicarbonate of 10. The decision is made to intubate the patient. Prompt cardiac consultation and evaluation demonstrate a critical coarctation of the aorta and appropriate treatment is initiated.
Coarctation of the Aorta. This case demonstrates a critical presentation of an infant with coarctation of the aorta. While the presentation and history were concerning for sepsis, several findings prompted the emergency department physician to request emergent cardiac evaluation: mottling of the lower extremities, inability to access peripheral veins, and inability to obtain blood pressures in the lower extremities. While these findings are subtle, they indicate the need to perform an echocardiogram.
Coarctation of the aorta has a 2:1 male predominance in the general population and represents roughly 6-8% of CHD in children. (See Figure 4.) The most common location for a coarctation is in the region where the ductus arteriosus connects to the aorta, typically just distal to the left subclavian artery. Infants with coarctation often have other cardiac defects, such as ventricular septal defect, atrial septal defect, aortic hypoplasia, or mitral valve anomalies.28 Coarctation can be associated with other genetic conditions, including Williams syndrome, DiGeorge syndrome, and Turner’s syndrome, where up to 18% of patients will have a coarctation of the aorta.29,30 Importantly, fewer than one in four patients with isolated coarctation requiring neonatal intervention are diagnosed prenatally, and clinical signs of coarctation can be subtle prior to a closing ductus arteriosus.31 This means that many patients with coarctation are discharged from the newborn nursery without diagnosis, and indicates the importance of keeping a high clinical suspicion in the emergency department when attending to a critically ill infant.
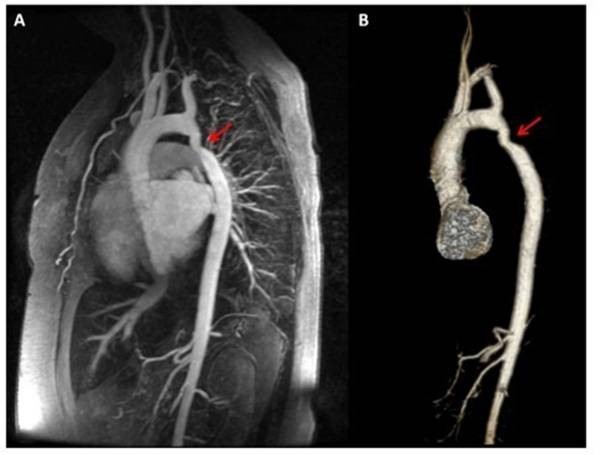
Figure 4. 3D Angiogram of Unrepaired Coarctation of the Aorta |
 |
|
Reprinted from: Juan LJ, Krieger, E, Valente AM, et al. Aortic dimensions on cardiovascular magnetic resonance imaging relate to pregnancy outcomes in women with coarctation of the aorta: A multicenter study. J Cardiovasc Magn Reson 2012;14(Suppl 1): O68. |
In the first two to six weeks of life, and more commonly between days three through seven of life, the ductus arteriosus begins to close, resulting in the development of aortic arch obstruction and reduced systemic perfusion. As a result, patients can begin to manifest signs of hypoperfusion to the lower body, diaphoresis with feeds, tachypnea, retractions, and cyanosis.32 Infants who present acutely during this period can have concurrent signs of renal failure, such as oliguria or anuria, mesenteric ischemia, and metabolic acidosis.31 Increased afterload on the left ventricle can result in left ventricular failure.28,33 These symptoms can progress to general circulatory shock.34
Common physical findings are a gallop and absent, delayed, or weak pulses in the lower extremities. There also may be a differential between upper and lower extremity pulse oximetry readings. A high blood pressure gradient between upper and lower extremities (> 20 mmHg) can be present. A chest radiograph often will be nonspecific but may show cardiomegaly with pulmonary venous congestion.
Treatment should consist of the infusion of prostaglandin E1 (PGE) to open and maintain the ductus arteriosus to improve systemic perfusion. Whenever possible, this should be done in consultation with a pediatric cardiologist.
A Note on Using Prostaglandin in the Emergency Department
PGE is directed at maintaining the patency of the ductus arteriosus. Early initiation of PGE is important in the course of CCHD because most critical obstructive lesions will be dependent on the ductus arteriosus. The most common risks of PGE include apnea and hypotension, so the infant should be monitored closely to observe for any hypercapnia or hypoxia.35 Intubation may be required or initiated in a prophylactic manner after the infusion has started. As described earlier, most of the treatments for critical congenital heart lesions in the infant are undertaken under the direction of the pediatric cardiologist and pediatric cardiac surgeon. However, for community emergency providers, PGE is a treatment that needs to be considered when there is a strong clinical suspicion for CHD. If transfer is required, a suspicion for cardiac disease should prompt initiation of PGE prior to transport.
Aortic Stenosis. Congenital aortic stenosis accounts for about 7.7% of all CHD.36-38 It occurs about four times more often in males than females, and has a strong association with Williams syndrome. Aortic stenosis presents with varying degrees of severity. The most pronounced form has only a pinhole opening through the aortic valve. There is a high rate of associated CHD defects, including coarctation of the aorta, hypoplastic left heart syndrome, mitral valve abnormalities, and ventricular septal defects.39
About 10% of patients with aortic stenosis will present critically in the newborn period. Typically, this is just after birth, or within the first one to two weeks as the ductus arteriosus begins to close. Critical aortic stenosis presents with cardiogenic shock and multiorgan dysfunction. As the ductus arteriosus closes, the left ventricle faces rapidly increasing resistance. The neonatal myocardium is immature and has low systolic and diastolic functional reserve in times of stress. Because of this, the neonatal heart cannot rely on modifying the stroke volume to maintain cardiac output, and instead relies on increasing the heart rate. Infants may begin to develop acute ventricular dilation rather than compensatory ventricular hypertrophy for this reason. This further increases wall stress and left ventricular end-diastolic pressure, increasing the myocardial oxygen demand. With only an elevated heart rate maintaining cardiac output, there is less time per cardiac cycle for diastolic coronary perfusion. This creates a cycle of increasing myocardial ischemia, cardiogenic shock, and multiorgan dysfunction, which will progress unless the obstruction is relieved.40
Those who have moderate aortic stenosis may present at any time during the first few months of life with symptoms of congestive heart failure, such as poor feeding, diaphoresis, failure to thrive, or respiratory distress. Most patients will have a normal blood pressure, but a narrow pulse pressure is present in more severe aortic stenosis. Physical exam findings may include a harsh systolic ejection murmur with an associated thrill; however, severe aortic stenosis may have a faint or absent murmur, in which case the peripheral pulses will be weak and difficult to palpate. Treatment in the emergency setting for infants with severe aortic stenosis should include rapid PGE infusion to help maintain adequate systemic and coronary perfusion. Neonates with cardiogenic shock should be started promptly on inotropic support after volume status is optimized. If vasoconstrictors are needed to augment organ and coronary perfusion pressure, care should be taken to weigh the utility of these agents with the risk of increased systemic vascular resistance, which further increases the pressure load on the left ventricle. Neonates may exhibit a unique combination of ventricular dysfunction with elevated systemic vascular resistance. Despite this, peripheral vasodilators should be avoided because they may lower coronary artery perfusion pressure.41
Neonates with critical aortic stenosis often have respiratory insufficiency related to cardiogenic shock. Intubation and mechanical ventilation can help with oxygen delivery and decrease metabolic demand related to the work of breathing. Sedation with narcotics and benzodiazepines can be useful to reduce metabolic demand, as can neuromuscular blockade and relative hypothermia. Stress corticosteroids should be considered in neonates who respond poorly to inotropic support. Glucose should be administered early because of the highly catabolic state of a neonate in cardiogenic shock. Definitive stabilization will require either surgical or catheter-based valvotomy.40,42
Pulmonary Stenosis. Pulmonary stenosis makes up about 10% of CHD cases.43 Pulmonary stenosis, like aortic stenosis, occurs with varying rates of severity dictating clinical presentation. Severe pulmonary stenosis will present within the first days to weeks with cyanosis. On exam, a systolic ejection murmur is typically heard, with or without a thrill. The longer and higher pitched the murmur, the more significant the degree of stenosis; however, the most severe types of critical stenosis may have a faint heart murmur due to severely restricted flow across the pulmonary valve. The most severe cases of pulmonary stenosis are dependent on the ductus arteriosus for blood flow to the pulmonary arteries and will present acutely in the first few days to weeks of life as the ductus is closing. Older infants with undiagnosed pulmonary stenosis can begin to develop right ventricular hypertrophy, which can cause additional dynamic obstruction of the right ventricular outflow tract.43 These patients can present with symptoms of respiratory distress, failure to thrive, cyanosis, or sudden death.
Chest radiographs typically will appear normal, and an electrocardiogram is often nondiagnostic.
Treatment in the emergency setting should consist of initiating PGE infusion to reopen the ductus arteriosus to supply pulmonary blood flow.
Cyanotic Lesions
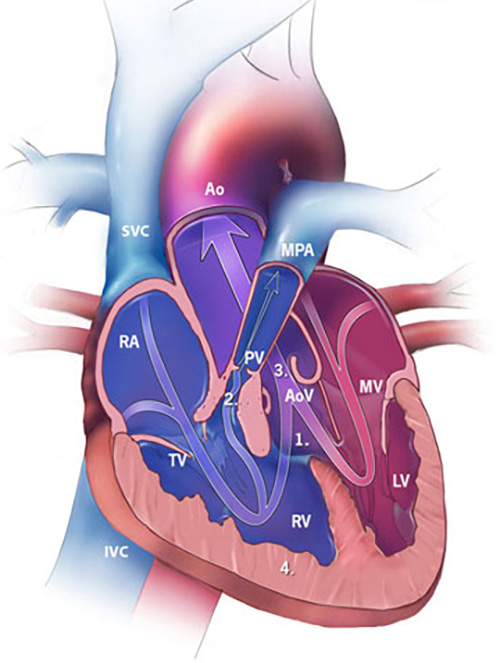
Tetralogy of Fallot. Tetralogy of Fallot represents between 3 and 10% of all CHD.44,45 Classically, it has been defined by four distinct anomalies: ventricular septal defect, overriding aorta, right ventricular outflow tract obstruction, and right ventricular hypertrophy. (See Figure 5.) The two abnormalities that are clinically significant are a ventricular septal defect large enough to equalize pressures between the two ventricles, and the right ventricular outflow tract obstruction. Right ventricular hypertrophy typically is a result of the degree of right ventricular outflow tract obstruction and large ventricular septal defect. The overriding aorta varies in position and degree. The clinical spectrum of this combination of disorders is broad, as are the presentations. The most severe type of tetralogy of Fallot includes pulmonary atresia, but tetralogy of Fallot with mild right ventricular outflow tract obstruction often is called “acyanotic” tetralogy of Fallot because of the near-normal oxygen saturation.
Figure 5. Diagram of Tetralogy of Fallot |
 |
|
RA: Right atrium; RV: right ventricle; LA: left atrium; LV: left ventricle; SVC: superior vena cava; IVC: inferior vena cava; MPA: main pulmonary artery; Ao: aorta; TV: tricuspid valve; MV: mitral valve; PV: pulmonary valve; AoV: aortic valve. |
The degree of cyanosis is related to the degree of right ventricular outflow tract obstruction. Infants with the most severe right ventricular outflow tract obstruction are dependent on the ductus arteriosus for pulmonary blood flow. They present with profound cyanosis during the first weeks of life as the ductus begins to close. Clubbing, dyspnea, or hypoxic spells also can be present in an older infant or child.46 A systolic murmur from pulmonary stenosis often is heard at the left sternal border. An infant with tetralogy of Fallot may present with periods of intense fussiness and cyanosis, which are typical of hypoxic spells (“tet spell”). These are demonstrated by rapid and deep respirations, irritability and prolonged crying, cyanosis (increased from baseline), and decreased intensity of the murmur. Severe spells can lead to seizures, flaccid tone, stroke, or death. During a hypoxic spell, the cerebral respiratory drive is increased, causing hyperpnea. Rapid deep breathing will cause an increase in systemic venous return, which forces more blood from right to left across the ventricular septal defect, making the hypoxia cyclical.
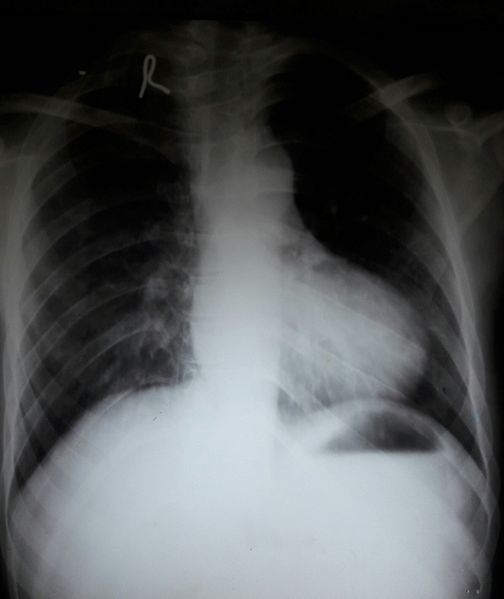
Most critical cases of tetralogy of Fallot present in infancy. An electrocardiogram will show right atrial dilation and right ventricular hypertrophy. A chest X-ray may demonstrate reduced pulmonary vascular markings, evidenced by lung fields that are more radiolucent. (See Figure 6.) It also can show the classic “boot-shaped” silhouette of the tetralogy of Fallot heart, but the heart will not be enlarged.
Figure 6. Chest X-Ray of Tetralogy of Fallot |
 |
|
Source: Medicalpal / Wikimedia Commons |
Treatment of tetralogy of Fallot with severe pulmonary stenosis or pulmonary atresia includes PGE to maintain patency of the ductus arteriosus.
An infant who presents with an acute hypoxic spell should be recognized and treated urgently. The approach to a hypercyanotic spell should begin with calming the child by relieving pain or anxiety. Parents can help by holding or calming the child. Concurrently, an effort should be made to increase systemic vascular resistance and reduce the right-to-left shunting. This can be done by holding the infant in a knee-chest position to increase the intraabdominal pressure. If there is no improvement within several minutes, IV access should be obtained. Morphine, which can be administered intramuscularly or subcutaneously, and midazolam, which can be given intranasally, can help the child remain calm while venous access is established, as well as help suppress the respiratory center to reverse the right-to-left shunting.47 Volume can be infused to optimize preload. Propranolol or esmolol may help prevent hypoxic spells by stabilizing peripheral vascular reactivity and allowing for greater ventricular filling time. In severe hypoxic spells with acidosis, treatment with sodium bicarbonate (1 mEq/kg IV) can help limit the activation of the central respiratory drive. If these measures fail, 1-3 mg/kg of ketamine in a slow IV push can increase the systemic vascular resistance and sedate the fussy infant, or 0.01-0.25 mg/kg of propranolol in a slow IV push can reduce the heart rate, reversing the spell.44
Transposition of the Great Arteries. Transposition of the great arteries represents 5-7% of CHD. There is a 60-70% male predominance.48 Transposition of the great arteries forms two parallel paths of circulation in which the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. Oxygenated blood returning from the lungs to the left side of the heart is pumped directly back to the lungs through the pulmonary artery, and deoxygenated blood is pumped back to the systemic circulation through the aorta from the right side of the heart. Thus, perfusing the systemic vasculature with oxygen-rich blood and returning oxygen-poor blood to the pulmonary vasculature is dependent on shunts between the two circuits. Mixing of blood through a patent ductus arteriosus or ventricular septal defect can help recirculate oxygen-poor blood to the lungs, but the majority of blood to the systemic vasculature is shunted at the level of an atrial septal defect.
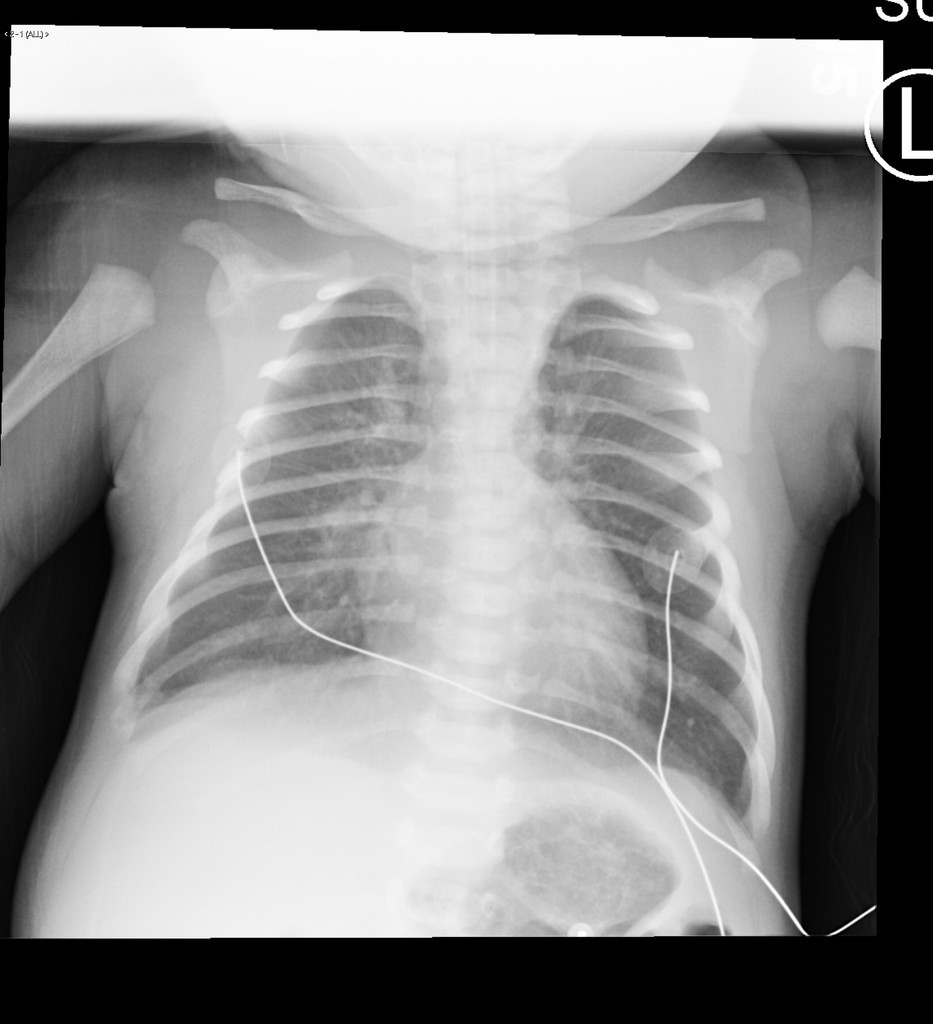
Infants are at a high risk of mortality with undiagnosed transposition of the great arteries. Without treatment, roughly 30% of infants with transposition of the great arteries will die within the first week, and ~90% will die within the first year.48 Infants with transposition of the great arteries typically present with cyanosis in the newborn period that is not responsive to oxygen. Hypoxia results in profound acidosis. Physical exam may reveal a loud single S2. Because of the orientation of the great arteries, the more anterior aortic valve is louder and obscures the sound of the pulmonary valve closure. A murmur may be present if there is associated pulmonic stenosis or another cardiac defect. A chest X-ray may reveal a narrow mediastinum, often described as having an “egg on a string” appearance. (See Figure 7.) An electrocardiogram often is nondiagnostic.49
Figure 7. Chest X-Ray of Transposition of the Great Arteries |
 |
|
Reprinted from Radiopaedia.org. |
Initial management includes rapid infusion of PGE to maintain patency of the ductus arteriosus. Metabolic acidosis, hypoglycemia, and hypocalcemia also should be treated aggressively if present. A patient with restrictive patent foramen ovale or with persistent hypoxia despite a patent ductus arteriosus may require balloon atrial septostomy within the first 24 to 48 hours of life.
Diagnostic Tools in the Emergency Department
We have discussed briefly the role of common diagnostic tools, such as electrocardiogram and chest X-ray, in the workup and diagnosis of critical CHD in the emergency department for each different congenital lesion. It is important to recognize the utility of these exams and their limitations. Electrocardiograms are often nondiagnostic for CHD, and rarely will influence ongoing management or workup. Chest X-rays are helpful, but should not be relied upon to diagnose or exclude CHD. Echocardiography remains the gold standard in diagnosis of CHD and should be requested when considering CHD in a critically ill infant.
Point-of-care cardiac ultrasound (echocardiography) is used increasingly by emergency providers and trainees, and is becoming more common among pediatric emergency medicine providers.50 The available evidence for the use of cardiac ultrasound has influenced the creation of guidelines in 2014 by the International Conference on Focused Cardiac Ultrasound. These guidelines strongly recommend that focused cardiac ultrasound should be used in the settings of pediatric patients in cardiac arrest, and to evaluate cardiac function, pericardial effusion, relative chamber sizes, valvular dysfunction, and volume status.51,52 It is important to note that the guidelines do not suggest that point-of-care focused cardiac ultrasound be used to diagnose CHD.53 Visualized abnormalities of the ventricular or atrial septum, cardiac function, poor or absent Doppler flow in the outflow tract, and dilated or hypertrophied chambers all can indicate the need for immediate follow-up with complete 2D echocardiography and consultation with a pediatric cardiologist. In settings where consultation with a pediatric cardiologist cannot be obtained immediately or transfer to a higher level of care is required, a point-of-care cardiac ultrasound that raises suspicion for obstructive or cyanotic heart disease in a critically ill infant, in combination with clinical suspicion, may indicate the need for PGE infusion. As with all point-of-care tests, the findings are limited by the experience and technical acumen of the operator, and any findings in a setting suspicious for CCHD — negative or positive — should be followed up promptly with complete 2D echocardiography.
Other rapid diagnostic tests may be performed at the bedside to help guide clinical decision-making. The role of pre- and post-ductal oxygen saturation has not been well evaluated in the emergency setting, but it can be incorporated easily into a routine assessment of vital signs by obtaining a transcutaneous oxygen saturation reading from the right hand (pre-ductal) and from either foot (post-ductal). Significant differences in the pre- and post-ductal oxygen saturation (> 3%) can be associated with an obstructive or cyanotic heart lesion and should indicate the need for a prompt cardiac workup.
Additionally, the hyperoxia test is helpful to distinguish a primarily respiratory cyanosis from cardiac cyanosis. After having the patient breathe 100% oxygen for 10 minutes, a post-ductal blood gas is obtained. If the PaO2 is greater than 150 mmHg, this suggests pulmonary disease. If the PaO2 value is below 150 mmHg, a cardiac cause of the cyanosis should be suspected and prompt workup should be initiated.54
Each of these tests has limitations and should be used only to guide the differential diagnosis, ongoing workup, and immediate management of patients with suspected CCHD. Initial history and physical examination should provide the clues to indicate the need for a cardiac workup.2 Clinical suspicion is the key to discovering undiagnosed CHD.
Conclusion
While most cases of CHD are identified through routine screening, as many as 20% of infants with CCHD may not receive a diagnosis prior to discharge from the newborn nursery.6 These infants can present acutely ill in the emergency department. Clinical presentation of shock, cyanosis, or respiratory distress can resemble that of other pediatric conditions, so it is important to maintain a high clinical suspicion for CHD in these patients.55 Recognition of patterns of presentation, including timing and risk factors, combined with a thorough history and physical exam can help with rapid diagnosis and management of these infants. For the emergency provider, a familiarity with the presenting patterns of CCHD is essential, as early diagnosis and intervention can lead to the best outcomes for these patients.
REFERENCES
- Triedman JK, Newburger JW. Trends in congenital heart disease. Circulation 2016;133:2716-2733.
- Fillipps DJ, Bucciarelli RL. Cardiac evaluation of the newborn. Pediatr Clin North Am 2015;62:471-489.
- Teitel D. Recognition of undiagnosed neonatal heart disease. Clin Perinatol 2016;43:81-98.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics - 2018 update: A report from the American Heart Association. Circulation 2018;137:e67-e492.
- Boneva RS, Botto LD, Moore CA, et al. Mortality associated with congenital heart defects in the United States: Trends and racial disparities, 1979-1997. Circulation 2001;103:2376-2381.
- Eckersley L, Sadler L, Parry E, et al. Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child 2016;101:516-520.
- Best KE, Rankin J. Long-term survival of individuals born with congenital heart disease: A systematic review and meta-analysis. J Am Heart Assoc 2016;5:pii:e002846.
- Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed 2008;93:F33-35.
- Brown KL, Ridout DA, Hoskote A, et al. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart 2006;92:1298-1302.
- Mahle WT, Newburger JW, Matherne GP, et al. Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the AHA and AAP. Pediatrics 2009;124:823-836.
- Massin MM, Dessy H. Delayed recognition of congenital heart disease. Postgrad Med J 2006;82:468-470.
- Chang RK, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med 2008;162:969-974.
- Acharya G, Sitras V, Maltau JM, et al. Major congenital heart disease in northern Norway: Shortcomings of pre- and postnatal diagnosis. Acta Obstet Gynecol Scand 2004;83:1124-1129.
- Glidewell J, Olney RS, Hinton C, et al. State legislation, regulations, and hospital guidelines for newborn screening for critical congenital heart defects - United States, 2011-2014. MMWR Morb Mortal Wkly Rep 2015;64:625-630.
- Liberman RF, Getz KD, Lin AE, et al. Delayed diagnosis of critical congenital heart defects: Trends and associated factors. Pediatrics 2014;134:e373-e381.
- Deshpande P, Baczynski M, McNamara PJ, Jain A. Patent ductus arteriosus: The physiology of transition. Semin Fetal Neonatal Med 2018;23:225-231.
- Wyllie JP, Gupta S. Prophylactic and early targeted treatment of patent ductus arteriosus. Semin Fetal Neonatal Med 2018;23: 250-254.
- Lee YS, Baek JS, Kwon BS, et al. Pediatric emergency room presentation of congenital heart disease. Korean Circ J 2010;40:36-41.
- Chapman SC. The decompensated neonate in the first week of life. Clin Pediatr Emerg Med 2016;17:134-139.
- Fisher JD, Bechtel RJ, Siddiqui KN, et al. Clinical spectrum of previously undiagnosed pediatric cardiac disease. Am J Emerg Med 2019;37:933-936.
- Mick NW. Pediatric cardiac disorders. In: Barton ED, Collings J, DeBileux PM, et al, eds. Emergency Medicine. 2nd ed. Philadelphia, PA: Saunders; 2014: 159-166.
- Brissaud O, Botte A, Cambonie G, et al. Experts’ recommendations for the management of cardiogenic shock in children. Ann Intensive Care 2016;6:1-16.
- Richards AA, Garg V. Genomics of congenital heart disease. Curr Cardiol Rev 2010;6:91-97.
- Yuan SM. Congenital heart defects in Williams syndrome. Turk J Pediatr 2017;59:225-232.
- Penny DJ, Vick GW 3rd. Ventricular septal defect. Lancet 2011;377:1103-1112.
- Crockett SL, Berger CD, Shelton EL, Reese J. Molecular and mechanical factors contributing to ductus arteriosus patency and closure. Congenit Heart Dis 2019;14:15-20.
- Backer CL, Eltayeb O, Mongé SC, et al. Shunt lesions part I: Patent ductus arteriosus, atrial septal defect, ventricular septal defect, and atrioventricular septal defect. Pediatr Crit Care Med 2016;17:S302-S309.
- Doshi AR, Chikkabyrappa S. Coarctation of aorta in children. Cureus 2018;10:e3690.
- Eckhauser A, South ST, Meyers L, et al. Turner syndrome in girls presenting with coarctation of the aorta. J Pediatr 2015;167:1062-1066.
- Noordman I, Duijnhouwer A, Kapusta L, et al. Phenotype in girls and women with Turner syndrome: Association between dysmorphic features, karyotype and cardio-aortic malformations. Eur J Med Genet 2018;61:301-306.
- Torok RD. Coarctation of the aorta: Management from infancy to adulthood. World J Cardiol 2015;7:765-775.
- Boris JR. Primary-care management of patients with coarctation of the aorta. Cardiol Young 2016;26:1537-1542.
- Dijkema EJ, Leiner T, Grotenhuis HB. Correction: Diagnosis, imaging and clinical management of aortic coarctation. Heart 2019;105:e6.
- Nguyen L, Cook SC. Coarctation of the aorta: Strategies for improving outcomes. Cardiol Clin 2015;33:521-530.
- Strobel AM, Lu LN. The critically ill infant with congenital heart disease. Emerg Med Clin North Am 2015;33:501-518.
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-2247.
- Eroğlu AG, Atik SU, Çinar B, et al. Echocardiographic follow-up of congenital aortic valvular stenosis II. Pediatr Cardiol 2018;39:1547-1553.
- Atik SU, Eroğlu AG, Çinar B, et al. Comparison of balloon dilatation and surgical valvuloplasty in non-critical congenital aortic valvular stenosis at long-term follow-up. Pediatr Cardiol 2018;39:1554-1560.
- Jashari H, Rydberg A, Ibrahimi P, et al. Left ventricular response to pressure afterload in children: Aortic stenosis and coarctation: A systematic review of the current evidence. Int J Cardiol 2015;178: 203-209.
- Affolter JT, Ghanayem NS. Preoperative management of the neonate with critical aortic valvar stenosis. Cardiol Young 2014;24:1111-1116.
- Kaza AK, Pigula FA. Surgical approaches to critical aortic stenosis with unicommissural valve in neonates. Expert Rev Cardiovasc Ther 2014;12:1401-1405.
- Kallio M, Rahkonen O, Mattila I, Pihkala J. Congenital aortic stenosis: Treatment outcomes in a nationwide survey. Scand Cardiovasc J 2017;51:277-283.
- Kwiatkowski DM, Hanley FL, Krawczeski CD. Right ventricular outflow tract obstruction: Pulmonary atresia with intact ventricular septum, pulmonary stenosis, and Ebstein’s malformation. Pediatr Crit Care Med 2016;17:S323-S329.
- Townsley MM, Windsor J, Briston D, et al. Tetralogy of Fallot: Perioperative management and analysis of outcomes. J Cardiothorac Vasc Anesth 2019;33:556-565.
- Downing TE, Kim YY. Tetralogy of Fallot. General principles of management. Cardiol Clin 2015;33:531-541.
- Karl TR, Stocker C. Tetralogy of Fallot and its variants. Pediatr Crit Care Med 2016;17:S330-S336.
- Montero JV, Nieto EM, Vallejo IR, Montero SV. Intranasal midazolam for the emergency management of hypercyanotic spells in tetralogy of Fallot. Pediatr Emerg Care 2015;31:269-271.
- Wernovsky G. Transposition of the great arteries and common variants. Pediatr Crit Care Med 2016;17:S337-S343.
- Sarris GE, Balmer C, Bonou P, et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. Eur J Cardiothorac Surg 2017;51:e1-e32.
- Guttman J, Nelson BP. Diagnostic emergency ultrasound: Assessment techniques in the pediatric patient. Pediatr Emerg Med Pract 2016;13:1-27;quiz 27-28.
- Via G, Hussain A, Wells M, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014;27:683.e1-683.e33.
- Klugman D, Berger JT. Echocardiography and focused cardiac ultrasound. Pediatr Crit Care Med 2017;17:S222-S224.
- Rosenfield D, Fischer JW, Kwan CW. Point-of-care ultrasound to identify congenital heart disease in the pediatric emergency department. Pediatr Emerg Care 2018;34:223-225.
- Singh Y, Chee YH, Gahlaut R. Evaluation of suspected congenital heart disease. J Paediatr Child Health (United Kingdom) 2015;25:7-12.
- Togănel R. Critical congenital heart diseases as life-threatening conditions in the emergency room. J Cardiovasc Emerg 2016;2:7-10.
Critical congenital heart disease (CCHD) is a significant cause of morbidity and mortality in children. When children with undiagnosed congenital heart disease (CHD) present acutely, the challenge of diagnosis and the importance of timely management can be daunting for any physician in an emergency setting. The children with the highest morbidity and mortality from critical congenital heart disease are infants younger than 1 year of age.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
