Deadly Pediatric Rashes
February 1, 2020
Reprints
AUTHOR
Brandi C. Barnes, MD, Pediatric Emergency Physician, Scottish Rite Children's Hospital, Atlanta, Egleston Children's Hospital, Atlanta
PEER REVIEWER
Aaron Leetch, MD, FACEP, Assistant Professor, Director Pediatric Emergency Medicine Residency, University of Arizona, Tucson
EXECUTIVE SUMMARY
• Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe, mucocutaneous, delayed hypersensitivity reactions characterized by a varying percentage of detachment of the epidermis from the basement membrane. Their classification is based on a continuum: An epidermis detachment of < 10% is SJS, detachment of 10-30% is SJS/TEN overlap, and > 30% is considered TEN. The rash is characterized by erythematous — and sometimes violaceous — patches, targetoid lesions, bullae characterized by a positive Nikolsky sign, and epidermal shear with light friction.
• Meningococcemia is a bacteremia caused by Neisseria meningitidis that may lead to severe sepsis and disseminated intravascular coagulation, and may have a high mortality rate even with appropriate identification and management.
• As of Jan. 1, 2010, cases of Rocky Mountain spotted fever (RMSF) are reported under a new category called Spotted Fever Rickettsiosis. In 2017, more than 6,248 cases were reported. It is difficult to discern how many of those cases specifically were RMSF because of the new reporting, but the CDC reports that in clinical reviews of RMSF cases, about 5-10% of cases are fatal, with the highest incidence between the ages of 5 and 9 years, and males are affected more commonly than females. The classic triad of fever, rash, and headache is seen only in about 3% of patients early in the course, but increases to around 70% later into the disease process.
• Toxic shock syndrome (TSS) is an acute, toxin-mediated illness characterized by fever, hypotension, multi-organ dysfunction, and diffuse rash with desquamation. Of the two types of TSS, menstrual TSS is associated more commonly with Staphylococcus aureus, and nonmenstrual TSS is associated more commonly with group A Streptococcus.
Rash is a common complaint in the emergency department (ED). Often, the pediatric rash is a benign, self-limiting condition that requires no intervention; however, there are occasions when rashes are true emergencies. Identifying these rare occasions is critical for the pediatric patient. This issue reviews and discusses some of the most common pediatric dermatologic emergencies and the ED approach to identification, diagnosis, and immediate evidence-based management of these conditions.
Case Presentations
A 10-year-old female presents to your ED with a rash. According to her mother, the rash started on her leg as a result of an insect bite, but continued to spread over several days. They went to the pediatrician, and the child was started on an antibiotic (unsure of the name). The rash seemed to get better initially, but then it started to spread. She does not have a fever, but the mother noticed her daughter’s eyes are very red. The patient says the rash hurts and she has a sore throat, and she cannot eat because her mouth is full of sores. She is tachycardic with a heart rate of 130. She has a few erythematous macules and patches, and targetoid lesions that coalesce into bullae. You slide your gloved hand over one, and the skin sloughs right off. What tests should you order, if any?
That same mother has a 5-year-old son who also has a rash. Per mom, the rash started around the same time for both of them. Is it contagious? He has had a fever for a few days. He will perk up during the day after getting some ibuprofen, but once the ibuprofen wears off, the fever returns and he is lethargic. He also seems more irritable than usual. She brought him in today because she noticed a diffuse rash all over his body that looks like blistering sunburn. He is afebrile right now, all of his vital signs are stable, and he is well appearing other than his skin. His rash is diffuse erythroderma with one or two areas of large bullae in one axilla that break and slough off easily when you apply gentle pressure. He is eating and drinking well. Where do you start? Can the patient go home or does he need to be admitted?
Introduction
Rash was one of the top 10 pediatric ED complaints in 2015.1 While most pediatric rashes are benign, on occasion, a dermatologic emergency will occur. It is critical that the emergency physician can identify the features of these potentially life- threatening emergencies.
This issue will discuss four major dermatologic emergencies and their differential diagnoses: Stevens-Johnson syndrome/toxic epidermal necrolysis; purpura fulminans caused by meningococcemia; Rocky Mountain spotted fever; and toxin-mediated syndromes, including toxic shock syndrome, necrotizing fasciitis, also known as necrotizing soft tissue infections, and staphylococcal scalded skin syndrome. The issue will take both a pedagogic as well as a clinical approach to these topics. Evidence-based medicine is used to evaluate, diagnose and manage each of these dermatologic emergencies from an ED perspective. Brief discussion of management beyond the ED will be broached to delineate novel and innovative theories discussed in the most up-to-date literature.
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
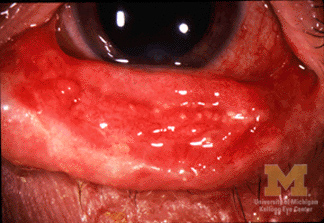
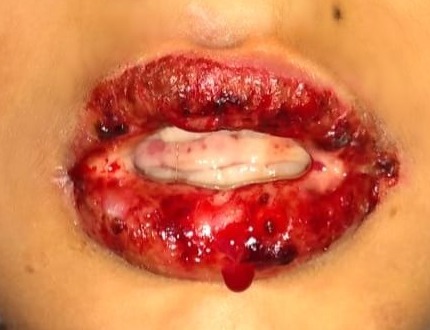
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe, mucocutaneous, delayed hypersensitivity reactions characterized by a varying percentage of detachment of the epidermis from the basement membrane. Their classification is based on a continuum. Epidermis detachment of < 10% is SJS (see Figure 1), detachment of 10-30% is SJS/TEN overlap, and > 30% is considered TEN (see Figure 2).
Figure 1. Stevens-Johnson Syndrome |
|
 |
 |
|
Left: Stevens-Johnson syndrome with conjunctivitis |
|
Figure 2. Toxic Epidermal Necrolysis |
 |
|
Image source: National Institutes of Health |
Epidemiology
In a recent study on SJS in the pediatric population, SJS was reported to have an incidence of 5.3, SJS/TEN of 0.8, and TEN of 0.4 cases per million age-adapted population.2 In children, the mortality rate is estimated to be 7.5%, with 95% having mucous membrane lesions.3 Another study suggests that in the HIV population, the annual incidence is approximately 1,000-fold higher than in the general population.4
Etiology and Pathogenesis
The most common culprit for SJS/TEN is a drug reaction, followed by a small number of cases caused by infection (namely herpes simplex virus and mycoplasma), with more than one-third of cases being idiopathic; however, understanding of the pathophysiology of SJS/TEN is rapidly evolving. Recent studies show that there is a genetic predisposition found on the human leukocyte antigen (HLA) allele that increases risk for this reaction, and varying HLA molecules are linked to specific drugs.5 Manifestations of the condition usually are present between one and three weeks after starting a medication. The most common drugs found to be associated with SJS/TEN are aromatic anticonvulsants, sulfonamide antibiotics, allopurinol, oxicam nonsteroidal anti-inflammatory drugs (NSAIDs), and nevirapine.6
Clinical Presentation
SJS/TEN usually presents with a prodrome of malaise, fever, cough, and/or sore throat followed by a very painful rash. The rash is characterized by erythematous — and sometimes violaceous — patches, targetoid lesions, and bullae with a positive Nikolsky sign. As the macules and patches evolve, dusky centers may be present, indicating epidermal necrosis. Involvement of the buccal, genital, and/or ocular mucosa occurs in more than 90% of patients.4
Diagnosis/Management
History taking is germane in coming to a swift diagnosis and identifying an offending agent. While there are histologic tests that can confirm keratinocyte apoptosis, these tests are neither practical nor are the results attainable in the ED setting. Laboratory tests including complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete metabolic panel (CMP), and urinalysis (UA) can help determine the degree of systemic involvement and if there is a concern for infection. A tool called the SCORe of Toxic Epidermal Necrolysis (SCORTEN) was developed to determine mortality risk in adults. Because of some of the criteria that need to be met, one study suggests that extrapolation to pediatric patients is ineffective.7 Another study argues that the development of a pediatric SCORTEN did not provide an advantage over the adult scoring system, which is applicable in pediatric patient risk determination.2 More research needs to be done to determine the use of SCORTEN for the management of TEN in the pediatric population.
There is no consensus on the ideal management for SJS/TEN; however, the overwhelming majority of studies in this review concur that removing the offending agent and providing supportive care are critical in managing this condition. Table 1 delineates the approach to managing SJS/TEN. Fluid replacement with crystalloid solution at 0.7 mL/kg/percentage of affected area, titrate to maintain a goal of 0.5-1 mL/kg/hour of urine output. Five percent albumin solution can be used in addition to crystalloid solution at a rate of 1 mL/kg/percentage of affected area,5 but it is not used often in the pediatric ED. Increasing the room temperatures to 30-32° C allows for decreased energy expenditure.5,7
Table 1. Approach to Managing Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis |
|
Early wound care has proven to increase positive outcomes.5-7 Although the optimal type of wound care has not been established, methods range from sterile saline-soaked gauze, to nonadherent dressings with triple antibiotic ointments, to petrolatum-impregnated or nanocrystalline gauze. Pain management should not be overlooked because pain increases metabolic activity, which is counterproductive to the resolution of this condition. When choosing an agent, take heed to not add an offending agent back into the mix, potentially worsening the patient’s condition (e.g., NSAIDs). While procuring the necessary consultants is not as critical in the ED setting as it is in the inpatient setting, it is important to be aware that this is a multidisciplinary condition. Based on the resources of the hospital, transfer to another facility may be needed if proper consultants are not available. At a minimum, they include dermatology and ophthalmology, but depending on progression of the condition, infectious disease and plastic surgery specialists may be needed. While intravenous immune globulin (IVIG), high-dose corticosteroids, tumor necrosis factor (TNF) alpha inhibitors, and cyclosporine are controversial and not initiated widely in the ED, they are therapies that may be adjuncts of potential benefit. More research is needed to confirm the positive benefit of these therapies. The consensus for disposition for patients with TEN is the burn unit, and opinions vary for SJS/TEN and SJS between the burn unit and intensive care unit (ICU).
Differential Diagnosis
The differential diagnosis for SJS/TEN includes staphylococcal scalded skin syndrome (SSSS), toxic shock syndrome (TSS), erythema multiforme, mycoplasma-induced rash and mucositis, bullous systemic lupus erythematosus, paraneoplastic pemphigus, and drug rash with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome (DIHS).
Meningococcemia
Meningococcemia is a bacteremia caused by Neisseria meningitidis that may lead to severe sepsis and disseminated intravascular coagulation (DIC), and it may have a high mortality rate even with appropriate identification and management. Purpura fulminans (PF) is a rapidly evolving syndrome of skin microvascular thrombosis and hemorrhagic necrosis. It is a sign of systemic disease rather than a disease unto itself. Three types of PF exist and include neonatal, idiopathic, and acute infectious. Neonates with PF usually have a hereditary deficiency of the anticoagulants protein C, protein S, and antithrombin III. Idiopathic PF is thought to be a postinfectious autoimmune disorder (relative deficiency of protein S), which often follows a febrile illness and later leads to rapidly progressive purpura. Acute infectious PF is the most common type. It manifests as a skin finding in the most severe septic patients as well as in necrotizing fasciitis (NF), with a predilection to certain infectious agents, specifically meningococcemia.
Epidemiology
According to the Centers for Disease Control and Prevention (CDC), in 2017, there were about 350 total cases of meningococcal disease reported (incidence rate of 0.11 cases per 100,000 persons). The rates of disease are highest in children younger than 1 year of age, with a second peak in adolescence. Among adolescents and young adults, those 16-23 years of age have the highest rates of meningococcal disease, which is in part due to residing in close quarters, such as barracks and college dorms. Invasive meningococcal disease (IMD) peaks during the months of November through March.8
Etiology/Pathogenesis
N. meningitidis is a Gram-negative diplococcus that often is found in the human nasopharynx. According to one review, the rate of colonization is much higher in the younger populations, which may be a contributing factor to the higher incidence of IMD in younger populations compared to adult populations.9 The primary cause of cardiovascular collapse from sepsis is due to peripheral circulatory failure. Endothelial cells increase their permeability as a result of increased circulating levels of cytokines TNF-alpha and IL-1 in response to bacterial endotoxins. Cytokines also play a role in suppression of myocardial contractility.
Clinical Presentation
Symptoms usually begin to manifest within two weeks of exposure. Nonspecific signs present initially: fever, malaise, and myalgias. In younger children, irritability and lethargy are seen more commonly. Neck stiffness is rare in children younger than 2 years of age, and a bulging fontanelle may be seen in children younger than 18 months of age.9 More classic signs are seen in older children, including nausea, vomiting, photophobia, neck stiffness, positive Kernig and/or Brudzinski signs, headache, agitation, and decreased level of consciousness.9,10 Septic shock is more common in children and progresses rapidly, with multi-organ failure and death often occurring within 24 hours.9 Adrenal hemorrhage and subsequent failure in the setting of meningococcemia is known as Waterhouse-Friderichsen syndrome.11,12 Leg pain, cold hands and feet, and abnormal skin color (mottling) are indicators of early sepsis marked by poor peripheral perfusion.9,10
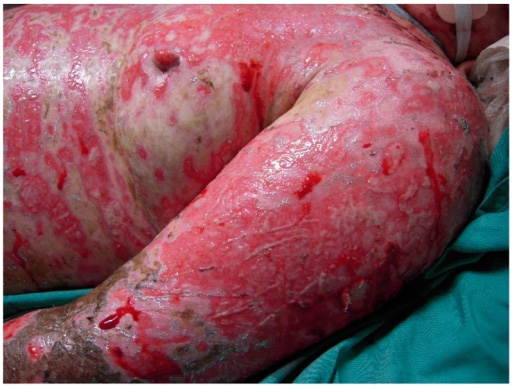
One of the most common symptoms associated with sepsis is a rash,9 which is pathognomonic for IMD.13 Some of the literature states the rash presents within 12-24 hours after initial symptoms.9,14 It can present as petechiae, purpura, and/or ecchymoses, which may progress to vesicles and bullae. In the early stages, the rash may resemble a viral exanthem: erythematous, maculopapular, nonpurpuric, nonpruritic, and often transient.10,14 As the rash progresses, irregular petechiae present on the limbs and trunk most commonly, but can be seen on the head, palms, soles, and mucous membranes.10 Ecchymoses and hemorrhagic bullae are formed as the lesions coalesce and may become gangrenous with progression of the disease.10 (See Figure 3.) PF often is the cutaneous manifestation of DIC.9
Figure 3. Pulpura Fulminans |
 |
|
Source: Wikimedia Commons |
Diagnosis/Management
Early identification of this disease is critical for improved prognosis and decreased morbidity and mortality. Positive identification of N. meningitidis in sterile body fluid is the gold standard to diagnose meningococcal infection; however, a few authors suggest that sensitivity for blood culture is about 50-60% and the sensitivity of cerebrospinal fluid (CSF) culture is 80-90%.12 Recent studies show that polymerase chain reaction techniques may yield a higher sensitivity than culture or Gram stain.10,12 While lumbar puncture (LP) is the ideal diagnostic test to confirm IMD, if the patient is hemodynamically unstable or shows signs of increased intracranial pressure, it may not be feasible. Additional recommended tests include CBC, CRP, ESR, CMP, magnesium, phosphorus, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, D-dimer, blood gas, blood culture, and lactate. A computed tomography (CT) scan may be warranted with depressed mental status to identify increased intracranial pressure.
Managing IMD requires both antibiotics and aggressive supportive care. The consensus on antibiotic therapy is a third-generation cephalosporin, such as ceftriaxone with meningitic dosing (100 mg/kg) either every 24 hours or divided into three doses per day. Penicillin G has been suggested by some studies at 300,000 U/kg/day;9,12,13 however, it is important to be aware of the antibiogram specific to the region in which the patient is living and being treated. Supportive care will vary based on the condition of the patient, but addressing airway, breathing, and circulation (ABCs) in the ED setting always is the best approach for management. Starting the patient on oxygen may help reduce metabolic demand. One source even suggests elective ventilation to control pCO2 to help improve cerebral perfusion via improved cardiac output.10 Fluid resuscitation is vital to avoid cardiovascular collapse. After administering 40-60 mL/kg of crystalloid fluids, inotropes should be considered for recalcitrant hypotension. Some studies suggest, if PF is present, also to give colloidal resuscitation, starting with fresh frozen plasma at 10-20 mL/kg every eight to 12 hours. Platelets (10-15 mL/kg) or cryoprecipitate (5 mL/kg) also may be needed.15 In several facilities, the standard of care in the treatment of meningococcal meningitis includes the administration of dexamethasone (0.15 mg/kg every six hours);16 however, the use of corticosteroids in pediatric patients in the setting of septic shock is not advised, except in the case of inotrope resistant shock. These patients require admission to the pediatric ICU (PICU).
Vaccines now exist for the prevention of many subtypes of N. meningitidis and have proven effective in the prevention of IMD.9,11,12
Differential Diagnosis
The differential diagnosis for PF includes heritable deficiencies of natural anticoagulants, Henoch-Schönlein purpura, necrotizing fasciitis, thrombotic thrombocytopenic purpura (TTP), idiopathic thrombocytopenic purpura (ITP), TSS, and Rocky Mountain spotted fever (RMSF).
Rocky Mountain Spotted Fever
RMSF is a tick-borne rickettsial disease seen almost exclusively in the Americas with a high mortality rate if left untreated. It is the most commonly fatal rickettsial disease in the United States.17 The initial clinical presentation can vary. Typically, the rash presents as erythematous and maculopapular, but it can evolve and is seen in only about 50% of cases.
Epidemiology
According to the CDC, RMSF is seen in all 48 of the continental U.S. states. The greatest geographic concentration is in North Carolina, Oklahoma, Missouri, Arkansas, and Tennessee, accounting for more than 60% of cases. Peak transmission is in May through August, with the exception of Rickettsia rickettsii. R. rickettsii is found year-round in Arizona and has been found to have a higher fatality rate. As of Jan. 1, 2010, cases of RMSF are reported under a new category called Spotted Fever Rickettsiosis (SFR). In 2017, more than 6,248 cases were reported. It is difficult to discern how many of those cases specifically were RMSF because of the new reporting, but the CDC reports that in clinical reviews of RMSF cases, about 5-10% of cases are fatal.18 According to the literature, most cases occur in pediatric patients, with the highest incidence between the ages of 5 and 9 years,19-21 and males are affected more commonly than females.19
Etiology/Pathogenesis
R. rickettsii is a Gram-negative, obligate, intracellular bacterium that is transmitted to humans via the bite of an Ixodes tick. Typically, the tick must be attached for four to six hours for transmission of the bacteria. It also can be transmitted via feces and being crushed (e.g., while being removed). It has a tropism for endothelial cells where it multiplies, causing focal endothelial proliferation and mononuclear cell infiltration and leading to red blood cell leakage and thrombosis of surrounding tissue, which can affect all organ systems.17,19,20,22 The bacteria also induce a procoagulant state, which is the pathogenesis for DIC (although it is quite rare).20
Clinical Presentation
The incubation period is about four to seven days but ranges from two to 14 days.17,19-22 Fever is the earliest and most common sign of infection and occurs in at least 97% of children with RMSF, often exceeding 102° F (38.9° C). Headache is present in 40-60% of children younger than 15 years of age and often is severe. About 90-95% of children have a rash at some point during the illness.17,20
The classic triad of fever, rash, and headache is seen only in about 3% of patients early in the course, but increases to about 70% later into the disease process.19,20
Altered mental status is present in about one-third of pediatric cases. Malaise, myalgia, abdominal pain, nausea, vomiting, and/or diarrhea are symptoms more commonly seen. One review suggests that the presence of bilateral calf pain is a classic symptom.21 Less frequently, photophobia, conjunctival injection, lymphadenopathy, hepatosplenomegaly, rhabdomyolysis, and periorbital and peripheral edema are noted. More rare but critical presentations include pulmonary edema, acute respiratory distress syndrome (ARDS), myocarditis, and even DIC.
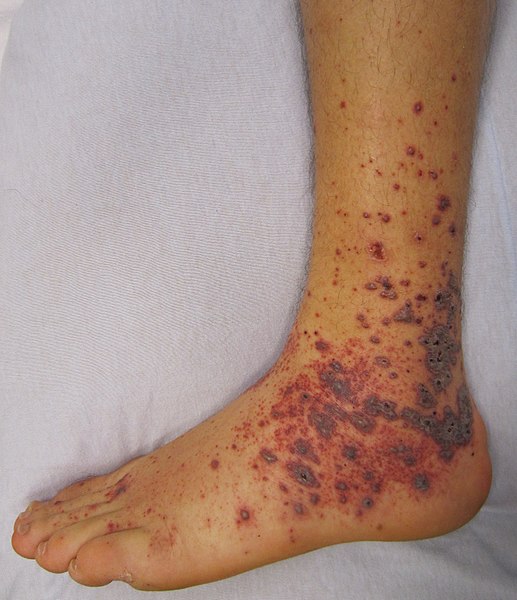
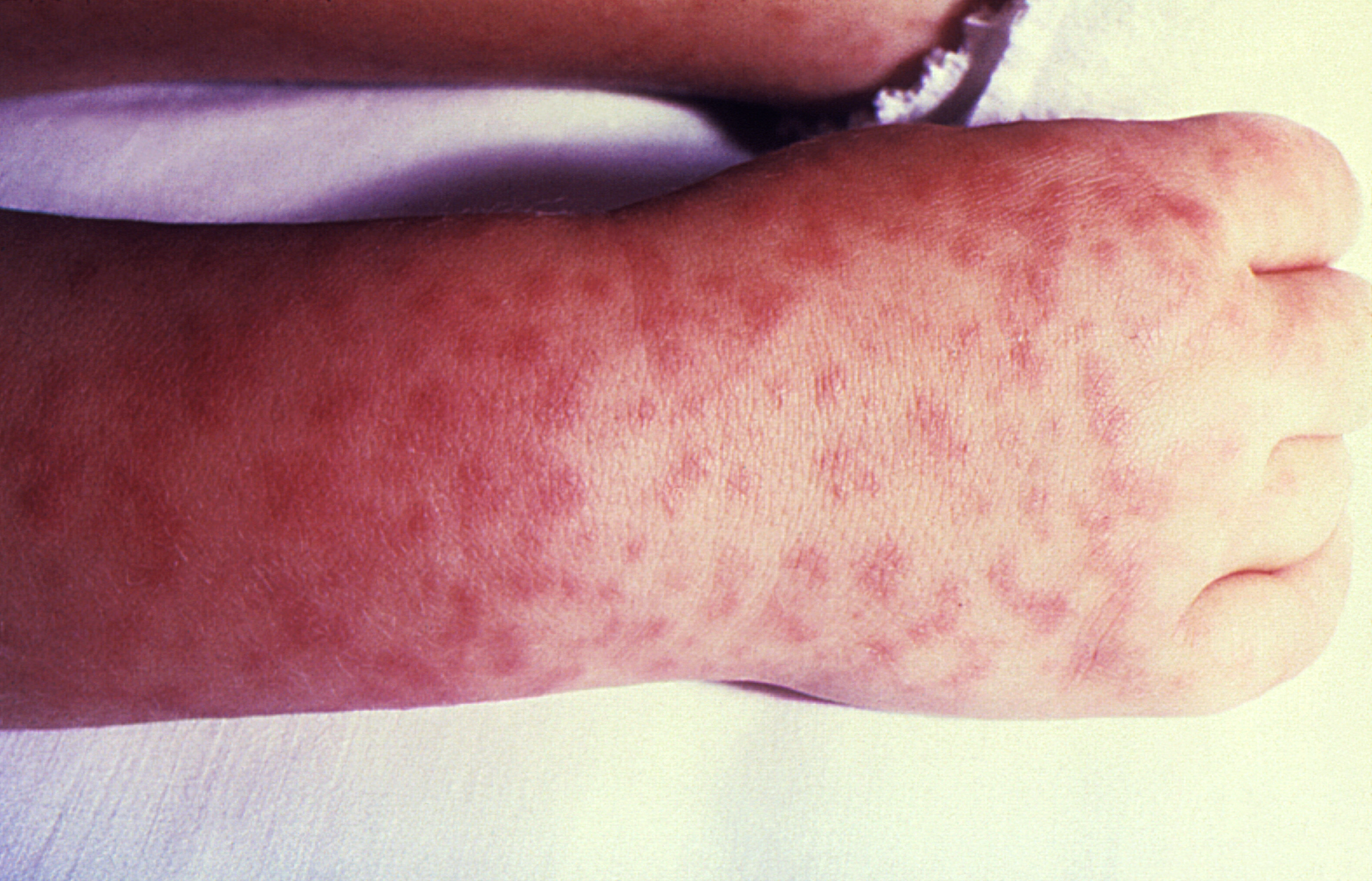
The rash typically presents as an exanthem between the second and fourth day of illness in children.17,19-21 It starts as small, blanching, pink macules on the ankles, wrists, and/or forearms. (See Figure 4.) As the rash evolves, it becomes maculopapular and expands centripetally to proximal extremities and the torso. The palms and soles become involved later in the course, but only affects about half of patients with RMSF. The face usually is spared. Petechiae can be seen in about 60% of pediatric cases about five days into the disease course. At this point, purpura and local areas of gangrene may be seen concurrently.10,17,19-21 Mucosal ulcers may be another cutaneous finding.
Figure 4. Rocky Mountain Spotted Fever |
 |
|
Source: Centers for Disease Control and Prevention |
Diagnosis/Management
The gold standard for diagnosis of RMSF currently is a serologic assay using indirect immunofluorescence antibody (IFA). However, this takes about seven to 10 days to get results, and management for suspected RMSF never should be delayed, so the diagnosis and decision to treat typically are made on clinical suspicion from the history and physical exam. Baseline labs should be obtained to discern the severity of disease and organ involvement. This includes CBC, CMP, CRP, ESR, PTT, aPTT, peripheral blood smear, creatine kinase, blood cultures, and LP.
The consensus on management is doxycycline (intravenous [IV] or oral). Although the use of doxycycline has been linked to permanent discoloration of teeth, many studies suggest that this is not likely to happen in the acute setting. The review of the literature suggests a dosing of 2.2 mg/kg per dose, up to a maximum adult dose of 100 mg per dose every 12 hours for a minimum of five to seven days, or until the patient is afebrile for more than three days, whichever is longer.11,19,21 Sulfonamides are strongly advised against, as researchers suggest mortality increases with their use.17,20,21 Researchers also suggest concomitant treatment with a third-generation cephalosporin, because there is significant overlap with meningococcemia. As with any condition, supportive care also is necessary. Provide pain management, fluid resuscitation, inotropes, and mechanical ventilation as required for support. If stable, these patients often can be managed on the pediatric hospitalist floor; however, the PICU may be needed in cases of severe illness.
Differential Diagnosis
The differential diagnosis for RMSF includes other, less fatal tick-borne illnesses (e.g., human monocytic ehrlichiosis, human granulocytic ehrlichiosis), human herpesvirus 6 (roseola), measles, Epstein-Barr virus, enteroviruses, secondary syphilis, drug eruptions, IMD, ITP, and TTP.
Toxin-Mediated Syndromes
Group A Streptococcus (GAS) and Staphylococcus aureus are two Gram-positive bacteria that can go from being benign colonizers to virulent and invasive infections. Their toxins in particular can cause self-limiting, localized skin infection and life-threatening systemic illness in a very short period of time. This section will focus on discussing three of the more dangerous syndromes caused by their toxins: TSS, NF or necrotizing soft tissue infection (NSTI), and SSSS.
TSS is an acute, toxin-mediated illness characterized by fever, hypotension, multi-organ dysfunction, and diffuse rash with desquamation. Of the two TSS types, menstrual TSS (MTSS) is associated more commonly with S. aureus, and nonmenstrual TSS (NMTSS) is associated more commonly with GAS. The incidence of NMTSS has become higher than MTSS because of a combination of patient education and the elimination of high absorbency tampons.23
NF is a rapidly progressive infection of the superficial fascia and subcutaneous tissue that can develop quickly into systemic disease and death. More recent literature uses the terminology NSTI to include all of the various iterations of NF. NSTIs typically are classified as polymicrobial (NSTI type I) and monomicrobial (NSTI type II). The pediatric population is affected more commonly by monomicrobial NSTI.
SSSS, also known as Ritter disease, is due to the exfoliative exotoxin of certain strains of S. aureus that causes severe blistering of the skin and desquamation, with systemic involvement in more severe infection. TSS, NF/NTSI, and SSSS will be discussed together because there is a considerable amount of overlap with pathology, presentation, and management of the diseases.
Epidemiology
Toxic Shock Syndrome. TSS has a suggested annual incidence of 1.5-11 per 10,000, with the highest incidence occurring in adults older than 45 years of age, but the second highest in children younger than 5 years of age, with the pediatric mortality rate ranging from 3-10%. According to the same review, the disease occurrence is higher in the winter and spring. Streptococcal TSS also seems to occur more commonly after viral infections, such as varicella, but also is seen with deeper sites of infection, such as penetrating injuries. Staphylococcal TSS is associated with postsurgical infection, burns, foreign bodies, soft tissue injury, and focal infections. They both can be seen in the setting of influenza, or without any apparent cause or source. Streptococcal TSS has higher rates of morbidity and mortality than staphylococcal TSS.24,25
Necrotizing Soft Tissue Infections. There are an estimated 1,000-1,500 cases of diagnosed NSTI in the United States annually at a rate of 0.04 cases per 1,000 per year.26 The incidence in children is less than in adults at 0.08 per 100,000 children.24 In a 29-year retrospective study from a single pediatric tertiary care center, NSTIs were responsible for 0.018% of hospitalizations.27 According to a study done by the Canadian Paediatric Surveillance Program (CPSP), males younger than 1 year of age had the highest disease burden, with 12 cases per million, vs. 3.2 cases per million for females younger than 1 year of age. There was about a 5% mortality rate in this study. These numbers include cases not related to GAS as well. Risk factors for the development of NSTIs in pediatric patients included chronic illness, trauma, surgery, and a recent infection with varicella.27 Other research demonstrated that varicella was associated with a 58-fold increased risk of acquiring invasive GAS, including both TSS and NSTIs.24
Staphylococcal Scalded Skin Syndrome. SSSS has an incidence of 0.09-0.56 cases per million people.25,28 The condition is seen most commonly in neonates and children younger than 5 years of age in the summer and autumn months.25
Etiology/Pathogenesis
Invasive GAS disease (including TSS and NSTI) is caused by streptococcal toxins that function as superantigens and activate T cells, leading to massive cytokine production. These cytokines mediate clinical effects, such as fever, rash, emesis, hypotension, tissue injury, and shock.
Clinical Presentation
TSS from both S. aureus and GAS present very similarly, with some exceptions. The only true way to distinguish the two etiologies is by isolating the bacteria, which is why the choice for empiric antibiotics should cover both. Signs and symptoms often are vague, and diagnosis frequently is missed on initial presentation. The patient can present early on with abrupt onset myalgias, generalized weakness, sore throat, and headache. Fever, malaise, vomiting, diarrhea, and abdominal pain also are early signs of illness.23,29,30 The rash seen with both GAS and S. aureus TSS typically presents as erythroderma, a diffuse, erythematous, blanching, macular rash resembling a sunburn. Mucous membrane involvement (e.g., conjunctiva, vaginal and oral mucosa) is not uncommon.23,29 A pruritic, maculopapular rash may develop during the first two weeks. Desquamation of the palms and soles may present one to three weeks after onset of the disease.23,29 Soft tissue necrosis is more likely to be seen in streptococcal TSS than staphylococcal TSS. Concern for NSTIs should be present when pain and vital signs are out of proportion to physical exam findings. Skin changes occur rapidly, which include blister formation and dusky appearance of extremities, as cutaneous ischemia develops.31 NSTIs are present more commonly on extremities; however, trunk presentation is seen more in children than adults.32 NSTI in the genital region specifically is referred to as Fournier’s gangrene. Tables 2 and 3 delineate clinical case definitions of staphylococcal and streptococcal TSS. Although these were designed for research, they have clinical implications. Systemic symptoms can progress very rapidly; neurologic signs of cerebral edema can present as confusion, seizure, and coma. Cardiopulmonary manifestations may include pulmonary edema, decreased cardiac contractility, hypotension, and pleural effusions. DIC also may be present in advanced disease.23,29,30
Table 2. Staphylococcal Toxic Shock Syndrome Clinical Case Definition |
|
Clinical Findings
|
|
Laboratory Criteria Negative results on following tests, if obtained:
|
|
Case Classification
|
|
Abbreviations: ULN, upper limit of normal; WBC, white blood cell; HPF, high power field; UTI, urinary tract infection; PLT, platelet; CNS, central nervous system; CSF, cerebrospinal fluid |
Table 3. Streptococcal Toxic Shock Syndrome Clinical Case Definition |
|
|
|
Case Classification
|
|
Abbreviations: ULN, upper limit of normal; serum Cr, serum creatinine; PLT, platelet; CSF, cerebrospinal fluid; DIC, disseminated intravascular coagulation; NF, necrotizing fasciitis |
In SSSS, the incubation period is typically one to 10 days25 and presents with similar vague systemic symptoms as TSS (i.e., fever, irritability, malaise). However, cutaneous findings are slightly different. The tender erythematous macular rash usually starts on the head and neck and progresses to the rest of the body. Tender flaccid bullae form, typically in the flexural areas, that are Nikolsky sign positive.23,25,28,33 The bullae enlarge and rupture easily to reveal a moist erythematous base, which gives rise to the scalded appearance.33 Although periorificial areas may be affected, mucous membranes are rarely affected; facial and periorbital edema may be present.28
Diagnosis/Management
As stated earlier, the only way to definitively diagnose staphylococcal vs. streptococcal infection is with identification of the bacteria, which is not practical in the ED setting. With that said, obtaining blood and wound cultures is important in tailoring management of the disease once bacterial identification and sensitivities result. For staphylococcal TSS, blood cultures are positive in less than 5% of patients. However, cultures from the site of infection, if identifiable, usually are positive.30,34 CBC, CMP, CRP, ESR, creatine kinase, PT, aPTT, and fibrinogen typically are needed to assess the severity of illness and organ system involvement.
CT and MRI of the site of infection also have been suggested as additional diagnostic tools to detect the extent of soft tissue involvement to discern if NSTI is present.30,35-37 It is important to note that imaging never should delay treatment if there is high suspicion of NSTI with a known site of infection.
Correction of hypotension with aggressive fluid resuscitation and inotropic support along with prompt empiric antibiotic therapy is essential to reduce the rate of morbidity and mortality in these conditions. A score called the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) was established in 2004. It uses the laboratory parameters of hemoglobin, white blood cell count, serum sodium, serum creatinine, serum glucose, and CRP to establish a score that predicts the likelihood of NF in a patient.38 This score has not been used in the pediatric population. However, studies that modify the score to be used in a special population like pediatrics may add a potentially beneficial supplemental tool in the diagnosis of NSTIs. LP and head CT may be required in the presence of altered mental status.
Antibiotic therapy typically is influenced by the antibiogram of the treating facility. As a general rule for staphylococcal infections, the recommended treatment is high-dose beta-lactamase-resistant antistaphylococcal penicillin (nafcillin/oxacillin 200 mg/kg/day divided in doses every four to six hours, with a maximum dosage of 12 g/day for either choice) and IV clindamycin (25-40 mg/kg/day, with a maximum dose of 900 mg/dose every eight hours).33,39 IV vancomycin (15 mg/kg/dose every six hours) is recommended if there is a high prevalence of methicillin-resistant S. aureus (MRSA) in the area. Clindamycin has been shown in several studies, in both GAS and S. aureus, to reduce the presence of exotoxins by inhibiting protein synthesis of bacteria, which reduces the superantigenicity of the toxin, diminishing subsequent cytokine cascade.29,30 Linezolid also has been shown to have this effect.29 For known GAS infection, administer IV penicillin G (200,000-400,000 U/kg/day in four to six divided doses).30,35 Some studies suggest more aggressive antibiotic management as a first-line empiric therapy when the bacterial source is unknown and MRSA has not yet been ruled out. Although they are less common in children, other microbial agents could include Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and/or Proteus mirabilis. Linezolid (10 mg/kg/dose, maximum dose of 600 mg/dose) or vancomycin26,29 and piperacillin-tazobactam or a fourth-generation cephalosporin for drug-resistant Gram-negative organisms along with clindamycin are recommended until susceptibilities result.26 Typical therapy duration is 10-14 days. IVIG (1-2 g/kg/day given once) has been suggested by several studies as an effective adjunct to the antibiotics. IVIG is believed to carry antistaphylococcal/antistreptococcal antibodies, which have been proven effective in reducing superantigenicity.30,36,37,40 Wound care should be considered early, if needed. New research is suggesting monoclonal antibody therapy as a promising adjunct to antibiotic therapy. In animal models, this murine monoclonal antibody neutralizes various superantigen activities in human peripheral blood mononuclear cells.41 The use of corticosteroids remains controversial throughout the literature and is not recommended at this time.
Aggressive surgical management, including debridement and fasciotomy, is critical in mortality reduction for patients with NSTIs.31,32,35 Involvement of the surgical team in the ED is critical in facilitating early intervention. In one retrospective study, surgical management reduced the mortality rate from 38% to 4%.42 Pain management also is critical for these patients. In SSSS, consider increasing the temperature of room if there is significant skin involvement. Mechanical ventilation may be required in severe systemic disease. Disposition of patients with toxin-mediated syndromes can vary from home in mild SSSS, to the PICU or burn center in severe cases.
Differential Diagnosis
The differential diagnosis includes meningococcemia, RMSF, Kawasaki disease, ehrlichiosis, measles, subcutaneous acute febrile dermatosis, and systemic lupus erythematosus. In the case of SSSS, SJS and TEN commonly are also considered as an initial diagnosis.
Summary
Pediatric rashes are a common complaint in the ED. The objective of this issue is to provide the tools necessary to identify, diagnose, and treat some of the dermatologic emergencies in the pediatric setting. Table 4 summarizes the rashes discussed in this issue, along with their diagnosis and management. It is important to pay attention to certain patient populations when driving your antibiotic management (e.g., immunocompromised, pregnant). Identifying specific signs may aid in the diagnosis (e.g., mucous membrane involvement, Nikolsky sign, rash distribution). Progression to septic shock in patients may be rapid and abrupt. It is important to anticipate complications and have a clear plan for managing these tenuous patients.
Table 4. Clinical Comparison of Diseases With Practical ED Approach |
||||
|
SJS/TEN |
Meningococcemia |
Rocky Mountain Spotted Fever |
Staphylococcal |
|
|
Prodromal/ presenting symptoms |
Malaise, fever, cough and/or sore throat, rash |
Younger children: Fever, irritability, lethargy Older children: Fever, photophobia, neck stiffness, headache, agitation, decreased level of consciousness, rash |
High fever, headache, rash, altered mental status, malaise, myalgia, abdominal pain, bilateral calf pain, photophobia |
Fever, irritability, malaise |
|
Rash description |
Erythematous/ violaceous macules and patches, targetoid lesions that coalesce into bullae characterized by a positive Nikolsky sign Palms (soles may be involved) |
Early rash is erythematous, morbilliform, non-purpuric. Progression into irregular petechiae. Further progression into ecchymoses and hemorrhagic bullae are formed as the lesions coalesce and may become gangrenous with progression of the disease. |
Early exanthem, small, pink macules that start on ankles, wrists, and/or forearms. Rash becomes maculopapular and expands centripetally to proximal extremities and torso; palms and soles become involved later. |
Diffuse erythroderma accented in flexural regions with progression to flaccid Nikolsky sign positive bullae. After rupture, erythematous base results. |
|
Mucosal involvement |
Yes, very common |
Yes, less common |
Yes |
No |
|
Inciting agent |
More common: drugs Less common: infection (HSV, mycoplasma) |
Neisseria meningitidis |
Rickettsia rickettsii |
Staphylococcus aureus |
|
Laboratory findings |
Elevated inflammatory markers, anemia, lymphopenia |
Elevated WBC with left shift, bandemia, anemia, hypoglycemia, hypocalcemia, hypomagnesemia, hypophosphatemia, coagulopathy, metabolic acidosis |
Leukopenia or leukocytosis, thrombocytopenia, hyponatremia, elevated aminotransferase levels, azotemia, elevated creatine kinase, coagulopathy (prolonged PT/aPTT) |
Elevated inflammatory markers, conjunctival hyperemia |
|
Physical exam findings other than rash |
Conjunctival hyperemia |
Signs of sepsis (fever, tachycardia, tachypnea, hypotension, hypoxemia), bulging fontanelle (infants), positive Kernig and/or Brudzinski signs |
Fever, lymphadenopathy, hepatosplenomegaly, conjunctival hyperemia, periorbital and peripheral edema |
Facial edema |
|
Antibiotic therapy |
Broad-spectrum if infection is suspected, acyclovir if HSV is suspected, macrolide if mycoplasma is suspected |
Third-generation cephalosporin |
Doxycycline AND third-generation cephalosporin (if unable to rule out meningococcal disease) |
Beta-lactamase-resistant PCN AND vancomycin (if MRSA unknown) OR First- or second-generation cephalosporin |
|
Adjunct therapya |
IVIG, corticosteroids, TNF-alpha inhibitors, plasmapheresis, cyclosporine |
FFP, platelets, cryoprecipitate, IVIG, corticosteroidsb |
None noted |
None noted |
|
a This does not include what is required of supportive care for sepsis (crystalloid resuscitation, inotropes, etc.). Abbreviations: SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; HSV, herpes simplex virus; WBC, white blood cell; PT, prothrombin time; aPTT, activated partial thromboplastin time; MRSA, methicillin-resistant Staphylococcal aureus; PCN, penicillin; IVIG, intravenous immune globulin; TNF-α, tumor necrosis factor alpha; FFP, fresh frozen plasma |
||||
The take-away from this article should be that rashes are managed in a stepwise approach, starting with identification from a thorough history and physical exam. Diagnostic tools, including laboratory tests and imaging, will help guide management via appropriate antibiotic therapy, management of ABCs, and appropriate use of consultants and proper disposition.
Case Conclusions
You obtain inflammatory markers, a CBC, and CMP on the 10-year-old. Inflammatory markers are elevated, her WBC count is normal, and her CMP shows signs of mild dehydration. You found that the antibiotic she was on was trimethoprim sulfamethoxazole. She complains of pain where the lesions are. She has about 5% skin involvement. She remains tachycardic, but she is still normotensive. The antibiotic is discontinued, and a normal saline bolus is given. She receives morphine for pain. You obtain blood cultures, start a broad-spectrum antibiotic, and consult the critical care team and dermatology. She is admitted to PICU, and dermatology will send their wound nurse to the ED to manage her lesions.
You obtain the same labs on the 5-year-old. His CRP and CBC are both mildly elevated, and CMP is unremarkable. He has since developed a fever and become more irritable. You decide to admit him to the hospitalist floor and start a beta-lactamase-resistant penicillin and vancomycin because MRSA has a high prevalence at your hospital.
REFERENCES
- McDermott KW, Stocks C, Freeman WJ. Overview of Pediatric Emergency Department Visits, 2015: Statistical Brief #242. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK526418/. Accessed Nov. 11, 2019.
- Lerch M, Mainetti C, Beretta-Piccoli T, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol 2018;54:147-176.
- Levi N, Bastuhi-Garin S, Mockenhaupt M, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A pooled analysis. Pediatrics 2009;123:e297-304.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis 2010;16:39.
- Schneider JA, Cohen PR. Stevens-Johnson syndrome and toxic epidermal necrolysis: A concise review with a comprehensive summary of therapeutic interventions emphasizing supportive measures. Adv Ther 2017;34:1235-1244.
- Papp A, Sikora S, Evans M, et al. Treatment of toxic epidermal necrolysis by a multidisciplinary team. A review of literature and treatment results. Burns 2018;44:807-815.
- Intong LR, Murrell DF. Chapter 78: Erythema Multiforme, Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. In: Irvine A, et al, eds. Harper’s Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011:911-918.
- CDC. Meningococcal disease. Surveillance. Available at: https://www.cdc.gov/meningococcal/surveillance/index.html. Published May 31, 2019. Accessed Dec. 27, 2019.
- Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: Epidemiology, clinical features and prevention. J Prev Med Hyg 2015;56:E121-124.
- Foust SN, Habibi P, Heyderman R. Chapter 55: Skin Manifestations of Meningococcal Infection. In: Irvine A, et al, eds. Harper’s Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011.
- Fidrocki D, Lutwick L. Fulminant meningococcemia. IDCases 2017;8:17-18.
- Takada S, Fujiwara S, Inoue T, et al. Meningococcemia in adults: A review of the literature. Intern Med 2016;55:567-572.
- Nadel S. Treatment of meningococcal disease. J Adolesc Health 2016;59:S21-28.
- Asif M, Quiroga L, Lagziel T, et al. A multidisciplinary approach to the management of severe purpura fulminans in a burn center: A case series. Cureus 2019;11:e5478.
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: Recognition, diagnosis and management. Arch Dis Child 2011;96:1066-1071.
- Theilen U, Wilson L, Wilson G, et al. Management of invasive meningococcal disease in children and young people: Summary of SIGN guidelines. BMJ 2008;336:1367-1370.
- McFee RB. Tick borne illness - Rocky mountain spotted fever. Dis Mon 2018;64:185-194.
- CDC. Epidemiology and statistics. Rocky Mountain spotted fever (RMSF). Available at: https://www.cdc.gov/rmsf/stats/index.html. Feb. 19, 2019. Accessed Dec. 27, 2019.
- Gottlieb M, Long B, Koyfman A. The evaluation and management of Rocky Mountain spotted fever in the emergency department: A review of the literature. J Emerg Med 2018;55:42-50.
- Woods CR. Rocky Mountain Spotted Fever in children. Pediatr Clin North Am 2013;60:455-470.
- Buckingham SC. Rocky Mountain spotted fever: A review for the pediatrician. Pediatr Ann 2002;31:163-168.
- Sexton DJ. Chapter 61: Rocky Mountain Spotted Fever and Other Rickettsial Infections. In: Irvine A, et al, eds. Harper’s Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011.
- Murray RJ. Recognition and management of Staphylococcus aureus toxin-mediated disease. Intern Med J 2005;35:S106-S119.
- Laupland KB, Davies HD, Low DE, et al. Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Ontario Group a streptococcal study group. Pediatrics 2000;105:E60.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: Evaluation, diagnosis, and management. World J Pediatr 2018;14:116-120.
- Miranda D, Bulger EM. Novel immune therapies in the management of streptococcal sepsis and necrotizing soft tissue infections. Surg Infect 2018;19:745-749.
- Fustes-Morales A, Gutierrez-Castrellon P, Duran-Mckinster C, et al. Necrotizing fasciitis: Report of 39 pediatric cases. Arch Dermatol 2002;138:893-899.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: Diagnosis and management in children and adults. J Eur Acad Dermatol Venereol 2014;28:1418-1423.
- Gottlieb M, Long B, Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: A review of the literature. J Emerg Med 2018;54:807-814.
- Chuang YY, Huang YC, Lin TY. Toxic shock syndrome in children: Epidemiology, pathogenesis, and management. Paediatr Drugs 2005;7:11-25.
- Jackson MA, Colombo J, Boldrey A. Streptococcal fasciitis with toxic shock syndrome in the pediatric patient. Orthop Nurs 2003;22:4-8.
- Zundel S, Lemaréchal A, Kaiser P, Szavay P. Diagnosis and treatment of pediatric necrotizing fasciitis: A systematic review of the literature. Eur J Pediatr Surg 2017;27:127-137.
- Patel GK, Finlay AY. Staphylococcal scalded skin syndrome: Diagnosis and management. Am J Clin Dermatol 2003;4:165-175.
- American Academy of Pediatrics. Toxic Shock Syndrome. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. 2018. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca (IL): American Academy of Pediatrics; 2000: 734-753.
- Seal DV. Necrotizing fasciitis. Curr Opin Infect Dis 2001;14:127-132.
- Nazreen J, Teach SJ. Necrotizing fasciitis. Pediatr Emerg Care 2011;27:1195-1199; quiz 1200-1202.
- Wilkins AL, Steer AC, Smeesters PR, Curtis N. Toxic shock syndrome - the seven Rs of management and treatment. J Infect 2017;74(Suppl 1):S147-S152.
- Bechar J, Sepehripour S, Hardwicke J, Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: A systematic review of the literature. Ann R Coll Surg Eng 2017;99:341-346.
- Millett CR, Heymann WR, Manders SM. Chapter 54: Pyodermas and Toxin-mediated Syndromes. In: Irvine A, et al, eds. Harper’s Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011:603-614.
- Norrby-Teglund A, Muller MP, Mcgeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scan J Infect Dis 2005;37:166-172.
- Kum WW, Chow AW. Inhibition of staphylococcal enterotoxin A-induced superantigenic and lethal activities by a monoclonal antibody to toxic shock syndrome toxin-1. J Infect Dis 2001;183:1739-1748.
- Bilton BD, Zibari GB, McMillan RW, et al. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: A retrospective study. Am Surg 1998;64:397-400; discussion 400-401.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
