Understanding Cannabidiol (CBD)
August 1, 2020
Reprints
AUTHORS
Cynthia Sheppard Solomon, BSPharm, RPh, FASCP, CTTS, NCTTP, Clinical Assistant Professor, Department of Internal Medicine
and Neurology, Wright State University Boonshoft School of Medicine, Dayton, OH
Glen D. Solomon, MD, MACP, CTTS, NCTTP, Professor and Chair, Department of Internal Medicine and Neurology, Wright State University Boonshoft School of Medicine, Dayton, OH
PEER REVIEWER
William Elliott, MD, FACP, Assistant Clinical Professor of Medicine, University of California, San Francisco
EXECUTIVE SUMMARY
• Cannabidiol (CBD) is used by a growing number of individuals for everything from anxiety to arthritis to seizures. A derivative from cannabis (marijuana) or hemp, it is a largely unregulated product that contains varying amounts of CBD.
• CBD has shown some effect in reducing the number of seizures in children with two rare syndromes. There is no clear evidence that it reduces seizures in other patients.
• CBD alters the cytochrome P450 enzymes similarly to grapefruit juice interfering with the metabolism of many drugs. Therefore, it may interfere with the metabolism of several drugs, including warfarin, antivirals, anxiolytics, antihypertensives, and antiepileptics.
• While CBD and other cannabinoids may be implicated in cannabinoid hyperemesis syndrome, it appears that delta-9-tetrahydrocannabinol (THC) is the common denominator found in medical histories of patients presenting with cannabinoid hyperemesis syndrome.
Cannabidiol is used by a growing population for many ailments. Although not typically an emergency drug, it has important interactions and a few side effects that can be seen in the emergency department. More importantly, because we are emergency providers, family, friends, and even casual acquaintances will ask our opinion of this new “miracle” drug. Therefore, we decided the topic was important enough to address in this issue.
— Sandra M. Schneider, MD, Editor
Introduction
Cannabis, also known as marijuana, and cannabis-derived products continue to be a controversial and confusing topic for both medical professionals and consumers.1 Cannabidiol (CBD) is one of two major constituents of marijuana and is the non-psychoactive component of cannabis. As its popularity has grown, questions about CBD from patients to their clinicians have increased dramatically. As a patient advocate interpreting the available evidence, the clinician needs to recognize how CBD differs from marijuana, what its clinical utility might be, and what its risks are.
Increasingly, the public views cannabis as harmless, often measured by how it is handled in the media.2,3 With more than 6.4 million Google searches for “CBD” in April 2019 alone, CBD and products containing CBD generally are not perceived by consumers as “harmful” compared to marijuana, alcohol, or electronic cigarettes.4,5 Numerous health and wellness products containing CBD often have been marketed without consideration for clinical efficacy, to the tune of $2 billion in sales per year.6 Sales projections as high as $16 billion to $24 billion by 2024-2025 concern those with knowledge of the weak science behind many health-focused commodities containing CBD.6
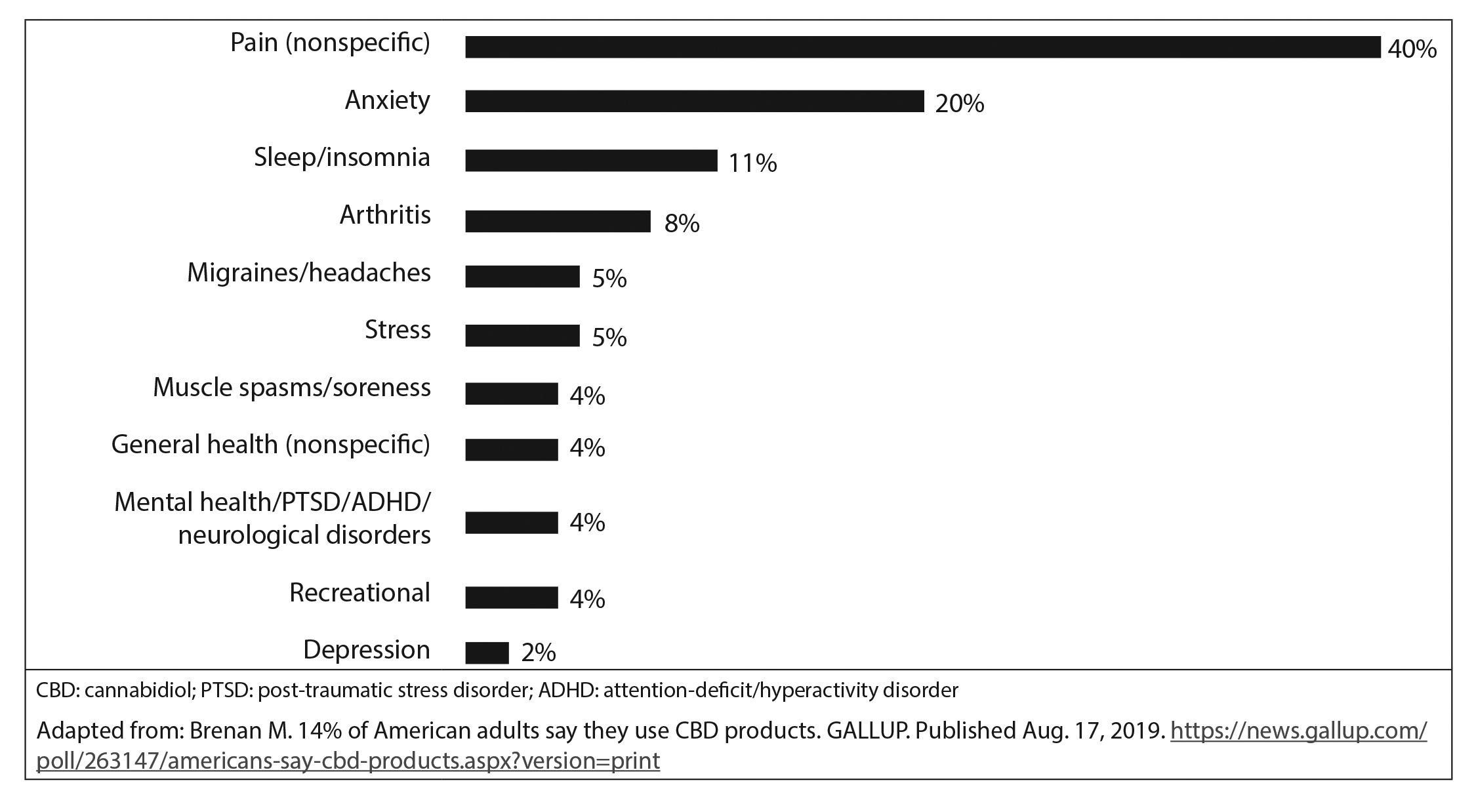
Clinicians must account for the well-demonstrated placebo effects, often related to symptoms, such as pain, anxiety, and sleep — which are key reasons for patients to seek out cannabis products.2,7-9 (See Figure 1.) Little evidence from large, randomized controlled trials demonstrates any clinical efficacy of CBD for medical claims, such as depression and post-traumatic stress disorder (PTSD), as promoted on CBD-labeled products. In a 2019 New York Times article that evaluated CBD’s benefits, Smita Das, MD, PhD, MPH, chair of the American Psychiatric Association’s Council on Addiction Psychiatry’s cannabis work group, stated that she does not recommend CBD for patients with anxiety, PTSD, sleep issues, or depression, and worries that falsely promoted substances may lead patients to more cannabis products.10 Many CBD products may be counterfeit or adulterated, contain substances not as labeled, contain no CBD, or not contain the type of CBD legally allowed in that specific product.1
Figure 1. Conditions or Purposes for Which CBD Is Used |
|
|
CBD: cannabidiol; PTSD: post-traumatic stress disorder; ADHD: attention-deficit/hyperactivity disorder Adapted from: Brenan M. 14% of American adults say they use CBD products. GALLUP. Published Aug. 17, 2019. https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx?version=print |
The Importance of Sources of Origin
The plant source from which CBD originates is Cannabis sativa L., representing a hybrid complex with three subspecies: Cannabis sativa, Cannabis indica, and Cannabis ruderalis.11 This hybrid complex is the source of a taxonomy controversy, hampering attempts to study the origins and the dispersal of this plant.11-13 (See “Sources of Cannabidiol.”)
Marijuana comes from the Cannabis sativa L. hybrid also. With its identifying psychoactive, euphoria-generating ingredient delta-9-tetrahydrocannabinol (THC), marijuana must contain concentrations of THC greater than 0.3%. THC is the ingredient responsible for the potency of each batch of marijuana. THC can be bred genetically in high concentrations, with potency levels in extracted hashish up to 90%.14,15 Marijuana contains THC and CBD as its two major components, in various ratios, and creates many varied effects with THC:CBD ratios that often are difficult to reproduce. A 1:1 THC:CBD ratio seems to be optimum for the least harm with some benefit. When THC content is high, CBD content is lower, and vice versa. At varying potencies, marijuana-derived CBD is found as the second most prevalent of the 400 constituents comprising marijuana and is the first of the three types of CBD discussed here. Marijuana remains a Schedule I controlled substance, regulated federally by the Drug Enforcement Administration (DEA) as an illegal drug. Despite this, nearly two-thirds of states have approved various forms of medical marijuana pursuant to state law.
The second type of CBD, also derived from marijuana, is the CBD specifically found in the Food and Drug Administration (FDA)-approved drug Epidiolex for add-on refractory treatment for two rare epileptic syndromes. This will be covered in detail later in the article.
Furthermore, in reference to products sourced from Cannabis sativa L., when the THC content of marijuana drops to 0.3% or less, the plant changes names, becoming a hemp plant and producing hemp. Officially, the federal 2018 Farm Bill identifies hemp as “the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.”16 Marijuana and industrial hemp, both closely related, had been classified similarly as Schedule I controlled substances for decades. Hemp is a high-fiber product used in many industries to make items such as cloth, rope, biodegradable plastics, and textiles. Hemp-derived CBD, the main component in the hemp plant, is the third type of CBD.17
In December 2018, with the passing of the 2018 Farm Bill, the federal status of hemp changed. Its previous designation as a DEA Schedule I controlled substance changed, shifting its supervision from the DEA to the U.S. Department of Agriculture (USDA).18 Industrial hemp, with extremely low concentrations of THC (0.3% or less) now can be transported in interstate commerce. Previously, it could not.
The 2018 Farm Bill does preserve the FDA’s authority over cannabis and cannabis-derived products, including hemp-derived CBD, following the rules of other FDA-regulated products. Any product marketed with a therapeutic claim must be approved prior to its introduction into interstate commerce — and no hemp-derived CBD products can carry therapeutic claims. The FDA does not consider the product’s source before exercising its regulatory authority, including whether a product originates from hemp or otherwise.18 Currently, the FDA prohibits the introduction of CBD products into the food supply or dietary supplements, even when derived from hemp. The FDA states that, because CBD is an active ingredient in the FDA-approved drug Epidiolex, incorporating CBD into the food supply or dietary supplements is illegal under the Food, Drug, and Cosmetic Act (FD&C Act).17,18 The FDA also clarifies that they will take action against those involved in illicit sales of cannabis products making therapeutic claims. Warning letters have been sent to companies illegally selling CBD-infused substances that claim to offer health benefits, such as preventing or treating a disease, or companies selling CBD-infused food products marketed as dietary supplements (violating the FD&C Act).18,19 One lawful way to introduce new products containing CBD into the United States is to create reproducible entities following the new drug approval rules of the FDA, the Investigational New Drug (IND) approval process, by studying it in randomized controlled trials, and proving efficacy for specific therapeutic purposes.
Still, the DEA considers cannabis (marijuana) or cannabis products sourced from marijuana, including those claiming to contain CBD and with concentrations of THC higher than 0.3%, to be Schedule I controlled substances. The DEA states that such substances have no currently accepted medical use and a high potential for abuse.17 State laws suggest that hemp-derived CBD (with THC content 0.3% or less) produced and marketed in that particular state is legal when found in products that do not promote therapeutic claims and that are labeled accurately for ingredients therein. These products would be found in local retail establishments, such as convenience stores and gas stations.
It is the hemp-derived CBD category of products that seems most fraught with fraud and scrutiny currently, since most people perceive this group of products to be helpful for almost everything and without adverse effects. Hemp-derived CBD products cannot be sold as food in most states and, federally, cannot be found in dietary supplements for ingestion. Also, the DEA Schedule I status of CBD makes products sold for pet consumption illegal. In most states, CBD must be manufactured within the state in which it is sold for any purpose and cannot legally be taken across state lines. CBD is not legally sold on the internet or internationally for transport back to the United States. If the product is marijuana-sourced, it is an illegal drug in accordance with FDA scrutiny. If it is hemp-derived, it cannot legally be transported across state lines.16 Unfortunately, many CBD products are not as they seem, nor are they lawful.
Medical Marijuana vs. Cannabidiol
It was hypothesized that medical marijuana might be an acceptable add-on therapy for seizure reduction in epilepsy, but research in this area has not panned out. Three consensus groups, including the National Academies of Sciences, Engineering and Medicine; Canadian Family Physician; and the Australian government do not recommend medical marijuana as a clinical therapy for epilepsy.20-22 On June 25, 2018, the FDA approved the first plant-derived, purified pharmaceutical-grade CBD medication comprised of a marijuana-derived active ingredient to treat rare, severe forms of epilepsy.23,24 The product is a pharmaceutical-grade version of CBD manufactured by GW Pharmaceuticals and is branded Epidiolex.12,17,23 It is indicated as an add-on treatment of intractable seizures in patients 2 years of age or older with the diagnosis of Dravet syndrome or Lennox-Gastaut syndrome — two rare epilepsy disorders. In clinical trials, adverse events included elevated liver enzymes, diarrhea, somnolence, and decreased appetite. Because of the significant effect on the liver, the FDA required that post-marketing studies be done in continued follow-up. Epidiolex is a reproducible entity, required to contain 0.1% or less of THC. Cannabidiol is a strong inhibitor of cytochrome P450 enzymes in the liver.25,26 As such, many medications are affected by the addition of CBD into the daily regimen.
In states where medical marijuana and/or recreational marijuana are legal, marijuana dispensaries carry genetic strains of marijuana that include those with higher CBD content, meaning THC is in lower amounts, but still greater than 0.3%, in compliance with its definition. In other words, at no time is it legal for CBD to be sourced from marijuana as a single ingredient, except for the prescription drug Epidiolex. When the CBD content in marijuana is higher, the THC level is lower. These higher CBD content strains still are considered marijuana, and in a state where medical marijuana is legal, must be recommended by a clinician who holds a “certification to recommend marijuana” in that state.14 There may be times when a clinician determines the patient should use a marijuana product with a higher amount of CBD vs. a higher amount of THC, but the clinician still would be recommending the use of medical marijuana and not hemp-derived CBD. It is important to reiterate that there is no evidence for CBD efficacy in any human studies except those associated with the two refractory epileptic syndromes for which Epidiolex is approved.
As therapies incorporating ingredients found in marijuana (cannabis), including CBD, are increasingly being investigated, potential clinical benefits, as well as the risks, must be recognized and appreciated before patients may achieve the best possible outcomes. Delving into the medical literature and looking at well-done studies on CBD is essential to medical decision-making regarding its appropriate safe use for patients.
Although there is little extensive research into the use of CBD by itself during pregnancy or while breastfeeding, the FDA states there is significant reason for concern.27 The FDA strongly advises that, during pregnancy and while breastfeeding, patients should avoid using CBD, THC, or marijuana in any form.
Challenges Associated with CBD
Making comparisons between CBD products in the few published studies is quite challenging because of the varied strengths and potencies of preparations promoted as CBD. The market is basically unregulated, and manufacturers often do not perform research to prove claims and verify potencies. Although preliminary evidence suggests the possibility of therapeutic value for several conditions, CBD is being promoted and recommended for a number of causes ahead of the medical evidence.28 Theorized effects of the molecular mechanisms of CBD cover the spectrum from anti-inflammatory to antipsychotic properties.26 Such properties have yet to be fully elucidated in human studies. From a pharmaceutical point of view, CBD has been touted as an “unusually interesting molecule” that has not yet proven the theories for the majority of conditions for which it might have effect.26 The lack of well-controlled, CBD-focused clinical trials is in part the result of the regulatory restrictions associated with Schedule I substances; the lack of CBD products produced under current, good manufacturing practices; and the variable quality, reproducibility, and purity of CBD from thousands of manufacturers.28
What Are the Effects of CBD and its Therapeutic Uses?
Routes of Administration
CBD most often is taken sublingually, by inhalation or vaporization, by ingestion, or applied topically. Oral administration delays the onset of action because of first-pass effects. The bioavailability of CBD is low by this route. Very few studies have been done evaluating the routes of administration of the various CBD preparations. Without standardized dosing levels, CBD-containing products can be unpredictable in reaching targeted effects. Topical application may not be absorbed sufficiently, and oral ingestion may be unpredictable in onset, duration, and metabolism, with numerous CBD formulations showing adverse effects without clinical benefit. CBD is quite lipophilic and can be affected by fatty meals when ingested.29
Mechanism of Action
The mechanisms of action of CBD are not well-defined and are very complex. Cannabinoid receptors (CBs) are highly prevalent in the human nervous system (CB1) and in immune cells (CB2).30 Unlike THC, CBD has a relatively small affinity to bind CB1 and CB2 receptors and may inhibit THC binding at CB1 receptors.31 At low concentrations, CBD has weak CB1 and CB2 antagonistic effects.30
In the nervous system, CBD may behave as a negative allosteric modulator of CB1, meaning that CBD does not activate the receptor directly, but it changes the efficacy and potency of THC and 2-arachidonoylglycerol at the CB1 receptor.30 Other potential actions of CBD include inhibition of gamma-aminobutyric acid (GABA), modulation of intracellular calcium by various transient receptor potential (TRP) channels, and modulation of tumor necrosis factor-alpha release or inhibition of adenosine reuptake.31 CBD also has activity at the serotonin 5HT1A receptor.30,32
In the immune system, CBD has been shown to inhibit neutrophil chemotaxis and proliferation. It may induce stimulation of arachidonic acid release, reducing prostaglandin E2 and nitric oxide production. CBD changes the expression of interleukins (ILs) by macrophages, reducing IL-12 while increasing IL-11.30 It is a direct agonist at the adenosine A2A receptor and the peroxisome proliferator-activated gamma (PPAR-gamma) receptor.33
Metabolism of CBD
CBD may interact with many different drug classes of medications and is associated with liver transaminase abnormalities. Research demonstrates a strong inhibitory effect of cytochrome P450 enzymes, especially CYP3A4, CYP2C19, CYP2C9, and CYP2D6.34-35 If CBD is taken while a patient is on other medications affected by the enzymes, CBD can block these enzymes, enabling more of the other medication to stay in the body at a higher level for a longer period of time. This is a similar effect to that seen when grapefruit juice interacts with medications. Warfarin, antivirals, anxiolytics (including benzodiazepines, antidepressants, and cardiac and hypertensive medications), and antiepileptics may be affected by this, causing serious problems for patients, depending on the CBD dose. Omeprazole, a modest inhibitor of CYP2C19, did not alter the plasma concentration of CBD in one study.34-36
For patients with epilepsy for whom the prescription CBD product Epidiolex is an add-on therapy to valproate, liver function tests may be abnormal.37 Since candidates for Epidiolex may be taking multiple antiepileptics concomitantly, close supervision of liver function and antiepileptic drug levels may be helpful. Topiramate, rufinamide, and N-desmethylclobazam (the active metabolite in clobazam) can increase in the body. Clobazam serum levels may need to be decreased with increased CBD doses.38
Therapeutic Uses
Despite commercial and testimonial promotion of CBD for various clinical conditions, there are very few clinical studies to support the therapeutic use of CBD. Extensive literature reviews, meta-analyses, and systematic reviews provide evidence for only one therapeutic use of CBD — add-on therapy for two childhood-onset epilepsy syndromes.
Epilepsy
The FDA has approved pharmaceutical-grade cannabidiol, CBD oil, for two childhood-onset epilepsy syndromes: Dravet syndrome and Lennox-Gastaut syndrome. Devinsky reported on a double-blind, placebo-controlled trial that compared pharmaceutical-grade CBD oil at a dose of 20 mg/kg/day with placebo as add-on therapy in 120 children and adults with Dravet syndrome. The study showed a statistically significant reduction in seizure frequency in the CBD group.39 It should be noted that two-thirds of patients in the CBD group were taking clobazam concurrently, and that CBD can increase the plasma concentration of clobazam and its active metabolite N-desmethylclobazam by three-fold to five-fold. It is uncertain if the observed improvement in seizure frequency was a direct effect of CBD or an increased plasma level of N-desmethylclobazam.31
A study by French et al evaluated CBD oil (20 mg/kg/day) as an add-on therapy for drop seizures in 171 patients with treatment-resistant Lennox-Gastaut syndrome.40 Compared with the placebo group, patients in the CBD group had a statistically significant reduction in the monthly number of drop seizures (44% vs. 22%). Another randomized controlled trial comparing two doses of CBD oil (20 mg/kg/day and 10 mg/kg/day) demonstrated a significant reduction in drop seizures with both doses vs. placebo (42% with 20 mg/kg/day vs. 37% with 10 mg/kg/day vs. 17% with placebo).31
A meta-analysis of 11 studies comprising 670 patients showed that 40% of the patients had more than a 50% reduction in seizure frequency after exposure to either hemp-derived CBD extracts or the pharmaceutical-grade, marijuana-derived CBD. Studies involving hemp-derived CBD extracts were done retrospectively and were not controlled trials. The meta-analysis reported that the dose of the pharmaceutical-grade CBD was four times higher than that of the hemp-derived CBD extracts. It was postulated that other phytocannabinoids in the hemp-derived CBD extracts might contribute to the higher potency. Additionally, patients treated with hemp-derived CBD extracts had fewer adverse events at the lower doses.41
In clinical trials, common adverse effects of CBD at the dose of 20 mg/kg/day included somnolence in 25% of patients, decreased appetite in 22%, diarrhea in 20%, and serum transaminase elevations in 16%. Side effects were dose-related. CBD did not cause intoxication or euphoria.42
Drug interactions are a concern for the use of CBD in epilepsy. Concurrent administration of moderate or strong CYP3A4 or CYP2C19 inhibitors can increase serum cannabidiol concentrations, increasing the risk of adverse effects. Concurrent use of drugs that induce CYP3A4 or CYP2C19 can decrease serum cannabidiol concentrations, reducing its efficacy. For example, it may be necessary to reduce the dosage of clobazam when it is co-administered with CBD.42
Psychiatric Disorders
Black et al performed a systematic review and meta-analysis of cannabinoids in mental disorders in 2019. They found no randomized controlled trials of CBD for depression. Two studies examined the effect of CBD in patients with social anxiety disorder and did not find a significant improvement in anxiety symptoms compared with placebo. No studies examined the efficacy of CBD in attention-deficit hyperactivity disorder or Tourette syndrome.8
In randomized controlled trials of CBD in psychosis, CBD did not improve total symptoms, positive symptoms, or negative symptoms compared with placebo or active comparators. CBD did not significantly improve cognitive or emotional functioning but did lead to improvement in global functioning compared with placebo.8
Arthritis
CBD is thought to have potential for arthritis treatment because it has theoretical analgesic, anti-inflammatory, and antioxidant effects. There are no controlled clinical studies in people with arthritis to support any claims of efficacy.43 A single placebo-controlled, randomized trial of synthetic transdermal CBD gel in 320 patients with knee osteoarthritis did not show a statistically significant reduction in pain scores, although secondary outcomes favored CBD.44
Pain
There are no published studies evaluating the analgesic effects of CBD in humans.33
Cancer
Although preclinical and animal models show promise for CBD in cancer treatment, this has not translated into evidence of benefit in humans. In preclinical models, CBD inhibited the survival of both estrogen-receptor-positive and estrogen-receptor-negative breast cancer cell lines.45 CBD also has been demonstrated to exert a chemopreventive effect in a mouse model of colon cancer.46
According to the National Cancer Institute, no ongoing clinical trials of cannabis as a treatment for cancer in humans were identified in a PubMed search.1 CBD oil extracts have been illegally promoted as potential cancer cures.47 These oils have not been evaluated in any clinical trials for anticancer activity or safety. Because CBD is a potential inhibitor of certain cytochrome P450 enzymes, CBD used concurrently with conventional therapies that are metabolized by these enzymes potentially could increase the toxicity or decrease the effectiveness of these therapies.48-50
Major known risks of CBD include liver toxicity (damage), extreme sleepiness, and harmful interactions with other drugs.27
Tips for Clinicians
Since there is much confusion for both patients and clinicians regarding CBD, it is important to review where one might expect to see various forms of CBD.
• Marijuana-derived CBD can be found in two places: 1) in the prescription drug Epidiolex or 2) in either medical or recreational marijuana varieties. If the CBD is sourced from marijuana, it is not the same thing as hemp-derived CBD (see the following legal definitions), and it is not legally allowed to be promoted as “hemp-derived CBD” as might be found in CBD-containing products sold on the internet or in gas stations, convenience stores, beauty shops, and other retail establishments. When it is found in these types of products, it is being sold illegally, since marijuana is a Schedule I illegal substance.
• When a product is labeled “hemp-derived CBD,” it can be sold legally in compliance with specific state laws in gas stations, convenience stores, pharmacies, and elsewhere without a prescription, if the product is not for ingestion (it cannot be sold legally as a dietary supplement or food, per the FDA). Such products cannot cross state lines for interstate commerce, which includes being sold on the internet for shipment from an out-of-state or international source. Many products sold as cosmetics for topical application may be counterfeit or adulterated with other ingredients, including
marijuana-derived CBD, which makes them illegal.
Because unpredictable effects may occur with dosing, potency, and route of administration, it is important for clinicians to be aware when individual patients are using CBD. Although a patient may perceive little harm from CBD, clinicians need to consider the patient’s entire medication regimen to protect him or her from liver abnormalities, drug interactions, and the risks of impaired driving or other conditions associated with substance use. Liver function tests and close monitoring may be valuable for patients with chronic conditions using CBD. For patients with epilepsy, CBD may interact with anticonvulsants already prescribed to them, including valproate.
Although CBD is not the psychoactive component in marijuana, CBD does have significant sedative and antipsychotic effects that may contribute to driving impairment, causing significant lethargy.37 These effects can be dose-dependent.34 Drug interactions with benzodiazepines potentially can cause increased sedation. CBD users also may fail drug tests when the product being consumed is of a higher THC content than allowed by law.
For patients with chronic medical conditions requiring multiple medications, CBD may require clinicians to adjust regimens accordingly. In some cases, clinicians may need to have a conversation with the patient about the risks of adding CBD to other medications.1 (See Table 1.)
Table 1. Tips for Clinicians |
|
Because many CBD oils often contain ingredients not as stated on the product label, dosing recommendations are difficult.30 A paucity of research exists evaluating topical absorption, oral or buccal administration, or the effects of vaping or smoking CBD preparations.2 Clinicians may need to adjust dosing based on clinical effects and adverse effects.
CBD — Helpful or Harmful?
CBD, as a plant ingredient, appears to have many varied effects, including the ability to reduce seizure frequency as add-on therapy in two childhood epileptic syndromes.50 Preclinical investigations have opened many avenues to those interested in the challenge of bringing drug products to market via new drug applications through the FDA. However, in human studies, little evidence has been published to support or advance the various preclinical theories touting the utility of CBD. Until such time as science catches up to the supposition, clinicians must stick with the evidence when they speak with patients or the public. Patients seeking answers to their ongoing challenges, whether chronic pain, generalized anxiety, or other chronic conditions such as Parkinson’s, PTSD, or Alzheimer’s, seek solutions that sometimes are too good to be true. Given the liabilities of CBD’s interactions with other drugs and herbs, along with the numerous deleterious unregulated CBD-labeled products of unknown provenance, clinicians need to have their eyes open to all possibilities.2
For children and pets, calls to poison control centers have skyrocketed with the increased availability of CBD and cannabis products of all sorts. Federally, CBD is not legal in food, nor is it legal in products for pet consumption. This does not stop unintentional exposures or the significant risk of excess sedation, drowsiness, liver toxicity, and many other acute problems related to children and pets accessing CBD as the result of improper storage.16
Acute Toxicity: Does CBD Play a Role in Cannabinoid Hyperemesis Syndrome?
For the physician evaluating patients in the emergency department setting, cannabis products can be associated with acute illness. Marijuana and cannabinoid-derived products containing THC can cause acute psychiatric symptoms, such as acute anxiety or psychosis, or worsening of chronic psychiatric conditions. Cardiovascular events, including myocardial infarction and ventricular dysrhythmia, also can occur. These often present after the use of edible cannabis.51
Cannabinoid hyperemesis syndrome (CHS) is a paradoxical condition of episodic intractable vomiting usually arising after long-term daily cannabis use, with complete abatement in a matter of hours or days only with cannabinoid abstinence.52-54 Often, vomiting is intense and overwhelming. While conventional antiemetic therapy such as ondansetron, promethazine, or metoclopramide is not effective, relief from nausea can be achieved intermittently by bathing in hot water.54 Patients may be unable to eat because of slowed gastric emptying and will require intravenous fluid replacement along with proton pump inhibitors, pain medications, and perhaps medications like haloperidol or topical capsaicin applied to the arm or abdomen. Patients often can undergo unnecessary tests, scans, and other procedures when clinicians and patients are unaware of its linkage to chronic marijuana (THC) use. Clinicians should be careful treating these patients with multiple medications, since there is significant opportunity for drug interactions, particularly with opioids and benzodiazepines, requiring tapering of one before adding the other.
Symptoms appear to involve episodic cyclic nausea and vomiting often accompanied by abdominal pain, with patients often presenting with having spent a lot of time in hot showers for symptom relief. Symptoms may include sweating, flushing, thirst, weight loss, and changes in body temperature. Complete relief occurs only with discontinuation of cannabinoids.51,52
Although CBD appears to have both antiemetic and pro-emetic dose-related effects, it appears that CHS patients will specifically have a history of chronic THC (marijuana) use.54 Both cannabidiol and cannabigerol have been implicated, along with THC, in the pathogenesis of CHS. It is unknown if certain varieties of botanical marijuana are more likely to produce CHS than others. The role of cytochrome P450 metabolism and genetic polymorphisms might increase susceptibilities of patients to CHS. It appears that chronic THC exposure is required for episodic hyperemesis.54
(For further information on adverse health effects associated with marijuana and medical marijuana use, see “Adverse Reactions to Cannabis and Cannabinoids” in the Oct. 15, 2018, issue of Emergency Medicine Reports.)
Conclusion
If patients seek CBD or if a clinician believes it is wise to recommend CBD for specific conditions, the bottom line is that one must use properly sourced CBD, and it must be used to treat conditions for which there is proven value. The “start low and go slow” premise is viable, along with close monitoring. Clinicians should stay informed, since much can change in the days and weeks ahead to clarify new clinical efficacy and reproducible drug entities with safe, repeatable dosing.
REFERENCES
- National Cancer Institute. Cannabis and Cannabinoids (PDQ) – Health Professional Version. https://www.cancer.gov/about-cancer/treatment/cam/hp/cannabis-pdq
- Haney M. Perspectives on cannabis research – barriers and recommendations. JAMA Psychiatry 2020; June 17. doi: 10.1001/jamapsychiatry.2020.1032. [Online ahead of print].
- Abraham A, Zhang AJ, Ahn R, et al. Media content analysis of marijuana’s health effects in news coverage. J Gen Intern Med 2018;33:1438-1440.
- Leas EC, Nobles AL, Caputi TL, et al. Trends in internet searches for cannabidiol (CBD) in the United States. JAMA Netw Open 2019;2;e1913853.
- POLITICO/Harvard T.H. Chan School of Public Health. Americans’ views on CBD products & marijuana for recreational use. Published November 2019. https://www.politico.com/f/?id=0000016e-3d52-ddf0-ad6e-bfd38a2a0000
- Miller RW. Everyone is asking Google about CBD. But does it actually work? USA Today. Updated Oct. 23, 2019. https://www.usatoday.com/story/news/health/2019/10/23/cbd-google-searches-cannabidiol-skyrocket-do-products-works/4062879002/
- Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: Patient characteristics, access, and reasons for use. Int J Drug Policy 2013;24;511-516.
- Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019;6:995-1010.
- Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the placebo effect: An affective appraisal account. Annu Rev Clin Psychol 2017;13:73-98.
- MacKeen D. What are the benefits of CBD? The New York Times. Updated Oct. 17, 2019. https://www.nytimes.com/2019/10/16/style/self-care/cbd-oil-benefits.amp.html
- Ren M, Tang Z, Wu X, et al. The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs. Sci Adv 2019;5:eaaw1391.
- Clarke RC, Merlin MD. Letter to the Editor: Small, Ernest. 2015. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Botanical Review 81(3):189-294. The Botanical Review 2015;2015;81:295-305. https://link.springer.com/article/10.1007%2Fs12229-015-9158-2
- Small E. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. The Botanical Review 2015;81:189-294.
- Ghosh TS, Van Dyke M, Maffey A, et al. Medical marijuana’s public health lessons — implications for retail marijuana in Colorado. N Engl J Med 2015;372:991-993.
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med 2014;370:2219-2227.
- Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). March 11, 2020. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
- Rubin R. Cannabidiol products are everywhere, but should people be using them? JAMA 2019;322:2156-2158.
- Hall, Render, Killian, Health & Lyman, PC. FDA clarifies position on CBD after passage of 2018 Farm Bill. Published Jan. 18, 2019. https://www.hallrender.com/2019/01/18/fda-clarifies-position-on-cbd-after-passage-of-2018-farm-bill/
- Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on signing of the Agriculture Improvement Act and the agency’s regulation of products containing cannabis and cannabis-derived compounds. Published Dec. 20, 2018. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-signing-agriculture-improvement-act-and-agencys
- The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies of Sciences, Engineering and Medicine 2017. Washington, DC. The National Academies Press.
- Australian Government Department of Health, Therapeutic Goods Administration. Guidance for the use of medicinal cannabis in Australia. December 2017. https://www.tga.gov.au/sites/default/files/guidance-use-medicinal-cannabis-australia-overview.pdf
- Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64:e78-e94.
- Abu-Sawwa R, Stehling C. Epidiolex (cannabidiol) primer: Frequently asked questions for patients and caregivers. J Pediatr Pharmacol Ther 2020;25:75-77.
- Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. Published June 25, 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
- Jiang R, Yamaori S, Takeda S, et al. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci 2011;89:165-170.
- Fernández-Ruiz J, Sagredo O, Pazos MR, et al. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol 2013;75:323-333.
- Food and Drug Administration. What you should know about using cannabis, including CBD, when pregnant or breastfeeding. Oct. 16, 2019. https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding
- Volkow ND. The biology and potential therapeutic effects of cannabidiol. Presented to the Senate Caucus on International Narcotics Control, June 24, 2015. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2015/biology-potential-therapeutic-effects-cannabidiol
- Kramer JL. Medical marijuana for cancer. CA Cancer J Clin 2015;65:109-122.
- Larsen C, Shahinas J. Dosage, efficacy and safety of cannabidiol administration in adults: A systematic review of human trials. J Clin Med Res 2020;12:129-141.
- Samanta D. Cannabidiol: A review of clinical efficacy and safety in epilepsy. Pediatr Neurol 2019;96:24-29.
- VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc 2019;94:1840-1851.
- Urits I, Borchart M, Hasegawa M, et al. An update of current cannabis-based pharmaceuticals in pain medicine. Pain Ther 2019;8:41-51.
- Wilkinson ST, Yarnell S, Radhakrishnan R, et al. Marijuana legalization: Impact on physicians and public health. Annu Rev Med 2016;67:453-466.
- Horn JR, Hansten PD. Drug interactions with marijuana. Pharmacy Times. Published Dec. 9, 2014. https://www.pharmacytimes.com/publications/issue/2014/december2014/drug-interactions-with-marijuana
- Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab Rev 2014;46:86-95.
- Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med 2018;378:
1888-1897. - Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015;56:1246-1251.
- Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017;376:2011-2020.
- French J, Thiele E, Mazurkiewicz-Beldzinska M, et al. Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): Results of a multi-center, randomized, double-blind, placebo controlled trial (GWPCARE4). Neurology 2017;88(Suppl). https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01766987/full
- Pamplona FA, da Silva LR, Coan AC. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front Neurol 2018;9:759.
- [No authors listed]. Cannabidiol (Epidiolex) for epilepsy. Med Lett Drugs Ther 2018;60:182-184.
- Fitzcharles MA, Niaki OZ, Hauser W, et al. Position statement: A pragmatic approach for medical cannabis and patients with rheumatic diseases. J Rheumatol 2019;46:532-538.
- Hunter D, Oldfield G, Tich N, et al. Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. Osteoarthritis and Cartilage 2018;26:S26.
- Shrivastava A, Kukontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther 2011;10:1161-1172.
- Aviello G, Romano B, Borrelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 2012;90:925-934.
- Food and Drug Administration. FDA warns companies marketing unproven products, derived from marijuana, that claim to treat or cure cancer. Published Nov. 1, 2017. https://www.fda.gov/news-events/press-announcements/fda-warns-companies-marketing-unproven-products-derived-marijuana-claim-treat-or-cure-cancer
- Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos 2011;39:2049-2056.
- Jiang R, Yamaori S, Okamoto Y, et al. Cannabidiol in a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet 2013;28:332-338.
- Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2017;2:139-154.
- Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: An observational study. Ann Intern Med 2019;170:531-537.
- Lapoint J, Meyer S, Yu CK, et al. Cannabinoid hyperemesis syndrome: Public health implications and a novel model treatment guideline. West J Emerg Med 2018;19:380-386.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: A case series of 98 patients. Mayo Clin Proc 2012;87:114-119.
- Pergolizzi JV Jr., Le Quang JA, Bisney JF. Cannabinoid hyperemesis. Med Cannabis Cannabinoids 2018;1:73-95.
Sources of Cannabidiol (CBD)
Cannabidiol (CBD) is not just one entity. There are three distinct variations that differ by source of origin and strength.
When marijuana is the source:
- CBD is a significant ingredient of marijuana, found in a mixture of more than 400 constituents. Delta-9-tetrahydrocannabinol (THC) and CBD are the major compounds responsible for marijuana’s effects. The CBD concentration may vary by batch in strength and potency, depending on the THC:CBD ratio, growing conditions, and genetics. Medical marijuana or recreational marijuana is federally categorized as a Schedule I illegal drug, although states approve individually whether medical marijuana or recreational marijuana is available for sale.
- CBD, with a THC content of 0.1% or less, is the Food and Drug Administration (FDA)-approved drug Epidiolex, a pharmaceutical-grade, reproducible oral solution (100 mg/mL) classified as a Schedule V controlled substance for the treatment of seizures associated with two rare, severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients 2 years of age and older. Epidiolex is the first FDA-approved drug containing a purified drug substance derived from marijuana. Epidiolex, as an add-on therapy, is effective in reducing the frequency of seizures. It was compared with placebo in three randomized, double-blind, placebo-controlled trials involving 516 patients with either Lennox-Gastaut syndrome or Dravet syndrome.1
When the origin is the hemp plant:
- CBD is found as the primary compound in hemp, and legally must contain 0.3% or less of THC. The hemp plant is a cousin of marijuana, although both originate with Cannabis sativa L.2 Products marketed as “hemp-derived CBD” are not always what they seem to be. Currently, these CBD-containing products fall into a neverland not consistent with or considered reproducible entities. CBD derived from hemp continues to be considered a Schedule I illegal drug. Currently, hemp is not a controlled substance, but CBD derived from it continues to be under the jurisdiction of the FDA and is illegal. State laws vary regarding the legality of CBD products. Manufacturers and distributors of products labeled as CBD often make a variety of claims without having to prove efficacy. The FDA does not consider them dietary supplements and, in fact, considers CBD in food or CBD products marketed to be ingested as illegal. Cosmetic CBD products manufactured and marketed locally from hemp-derived CBD may be legal as long as they are not transported in interstate commerce. Therapeutic claims made for hemp-derived CBD-containing products are not allowed. In a recent study of 84 products evaluated for CBD labeling accuracy, only one-third (30%) were labeled accurately, while the rest were over-labeled, under-labeled, or did not contain CBD at all.3
REFERENCES
- Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. Published June 25, 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
- Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products including cannabidiol (CBD). March 11, 2020. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
- Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017;318:1708-1709.
Cannabidiol is used by a growing population for many ailments. Although not typically an emergency drug, it has important interactions and a few side effects that can be seen in the emergency department.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.