Nonpenetrating Ocular Trauma Part I: Severe Vision Threats
April 15, 2021
Reprints
AUTHORS
Dustin B. Williams, MD, FACEP, Associate Professor, Program Director, Emergency Medicine Residency, University of Texas Southwestern, Dallas
Melissa Smith, MD, Assistant Professor, Assistant Program Director, Emergency Medicine Residency, University of Texas Southwestern, Dallas
Aman Pandey, MD, Senior Resident, Department of Emergency Medicine, University of Texas Southwestern, Dallas
Larissa Velez, MD, Professor of Emergency Medicine, Vice Chair for Education, University of Texas Southwestern, Dallas
PEER REVIEWER
Catherine Marco, MD, FACEP, Professor, Department of Emergency Medicine, Wright State University, Dayton, OH
EXECUTIVE SUMMARY
- Consider eye injuries in patients with facial and intracranial injuries.
- Avoid manipulating the globe until it has been confirmed that there is not a ruptured globe.
- Do not delay lateral canthotomy and cantholysis when a retrobulbar hemorrhage with an orbital compartment syndrome is identified.
- Consider orbital apex syndrome if there is both impaired vision and impaired globe motility.
- Ocular ultrasound, also referred to as a B-scan, can be useful in distinguishing between traumatic vitreous hemorrhage and retinal detachment.
Epidemiology of Eye Trauma
According to the National Center for Health Statistics’ Health Interview Survey, there are 2.4 million eye injuries per year in the United States. The World Health Organization Programme for the Prevention of Blindness suggests that, worldwide, about 55 million eye injuries occur each year that restrict activities for more than one day. In the United States, the United States Eye Injury Registry (USEIR) and the National Institute for Occupational Safety and Health (NIOSH) both collect ocular injury data in a standard fashion. However, there is not a standardized international template for reporting on eye injuries, so comparisons between different countries is difficult.
This article will focus on nonpenetrating eye injuries that are severe threats to vision. Part II will discuss potential vision threats and special populations.
Patients younger than 30 years of age are at the most risk. Falls are the No. 1 reason for blunt ocular trauma, with most falls happening in those 60 years of age or older. These falls occur from slipping, tripping, or falling down the stairs.1 Fighting and various types of assault are the second most common cause of ocular trauma overall, but they are the leading cause in people ages 10-59 years.1
About one-third of blunt ocular injuries are job-related. Injuries related to sports and other recreational activities also are common. Hockey, baseball, basketball, and racket sports (tennis, badminton) pose the highest risk.2,3 Toy guns and paintball guns are a common cause of injury widely reported in the literature.4-7 In children, being struck by an object or by a person is the number one cause for blunt eye trauma in those younger than 10 years of age, and motor vehicle collisions (MVCs) are the second most common cause.1 In children, blunt eye injury also can be a presentation of nonaccidental trauma (NAT).6
Definitions and Classifications of Ocular Injuries
The Birmingham Eye Trauma Terminology System is a standard terminology used to describe and share eye injury data.8 (See Table 1.) This is important not only in data collecting, but also in offering a common language to communicate with consultants in the emergency department (ED).
Table 1. Birmingham Eye Trauma Terminology Terms |
|
|
Term |
Definition |
|
Eye wall |
The sclera and cornea that surround the eye contents |
|
Closed globe injury |
Absence of a full-thickness wound of the eye wall |
|
Contusion |
No full thickness injury, generally from blunt trauma |
|
Lamellar laceration |
Partial thickness injury of the eye wall, generally from a sharp injury |
|
Open globe injury |
Full-thickness wound to the eye wall |
|
Rupture |
Full-thickness wound of the eye wall, usually from a blunt injury, typically with prolapse or extrusion of global contents |
|
Laceration |
Full-thickness wound of a single eye wall, from a sharp object |
|
Penetrating injury |
Single laceration on the eye wall |
|
Intraocular foreign body |
Retained intraocular object that caused an eye wall laceration |
|
Perforating injury |
Both entrance and exit wounds to the eye wall, caused by a sharp object or missile |
The Ocular Trauma Scoring (OTS) system predicts the six-month outcome after open globe trauma. It is only valid for mechanical injuries to the eye, and it excludes chemical, electrical, and thermal injuries. The score is based on initial visual acuity and the presence of globe rupture, endophthalmitis, perforating injury and/or retinal detachment, and a relative afferent pupillary defect (RAPD).8-10 Scores range from 1 (worst) to 5 (best). The predictive accuracy of the OTS for visual outcome is 80%. While this score allows for better communication between clinicians,11 its limitations include no consideration of concomitant injuries that may affect the overall outcome. The scoring system does not include imaging data that may help refine the injury.
History
The three most useful historical aspects (“red flags” for serious injury) when evaluating an eye injury patient are: direct ocular trauma, eye pain (and pain with eye movement), and a new visual impairment (including a visual field impairment).12
Obtain details about the injury mechanism. High-energy mechanisms include:
- sports;
- work activities;
- hobbies and other recreational activities that involve striking or grinding;
- falls, especially in geriatric patients;
- MVCs — note the speed, use of seat belts, and presence of air bags
- fights and altercations — note the type of weapon used;
- toy guns or paintball guns — ask about eye protection used.
The timing of the injury and the timing of the onset of visual impairment also are very important. For example, the prompt identification and management of retrobulbar hematomas with concomitant ocular compartment syndrome is critical to good visual outcomes.
The clinician should inquire if there were any prehospital treatments or interventions. For example, in chemical injuries, prompt and thorough decontamination and eye irrigation are critical.
The use of any anticoagulant also is relevant to the history, since the use of these medications lowers the threshold for bleeding in the setting of trauma.
Eye Examination
Address any life threats first. Once any life or limb threats have been addressed, perform a detailed examination of the face and the eye. Blunt eye injuries can be accompanied by intracranial injuries and facial bony trauma. The eye should be manipulated as little as possible until the clinician is sure that there is not a ruptured globe. (See Figure 1.) If the integrity of the globe is uncertain, or if the physician diagnoses a ruptured globe, an eye shield should be applied to prevent further damage. The clinician should manage pain and nausea, since both can worsen intra-orbital pressure.
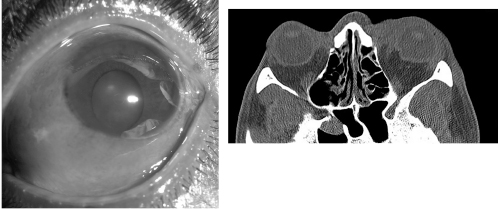
Figure 1. Ruptured Globe |
|
Ruptured globe with subconjunctival hemorrhage and deformed pupil (left). CT of ruptured globe on the left with deformed shape (right). |
|
Image courtesy of: J. Stephan Stapczynski, MD |
If there is a history of a chemical eye exposure, irrigation must occur as soon as possible. The rest of the exam should be delayed until after irrigation is complete.
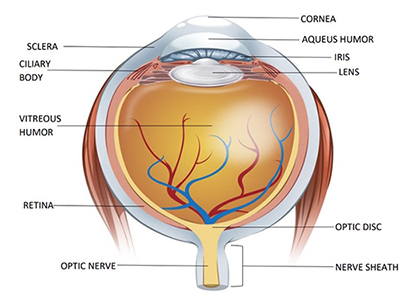
Knowledge of basic eye anatomy is important to properly identify injuries. (See Figure 2.) Table 2 lists all of the relevant portions of the eye examination, along with important findings to note.
Figure 2. Eye Anatomy |
 |
|
Image courtesy of: Apoorv Pandey |
In general, the ED goals of initial assessment and management are to assure there is not a ruptured globe, to identify emergencies that need emergent ophthalmologic consultation, and to identify any conditions that need emergent intervention. (See Table 3.)
Table 2. Eye Examination13-16 |
|
|
Standard Eye Examination |
Critical Findings |
|
Visual acuity |
|
|
Eyelids |
|
|
Extra-ocular movements |
|
|
Conjunctivae |
|
|
Pupils |
|
|
Visual fields |
|
|
Cornea |
|
|
Anterior chamber |
|
|
Lens |
|
|
Vitreous |
|
|
Retina and macula |
|
|
Adjuncts to the Examination |
|
|
Intraocular pressures |
|
|
Wood’s lamp |
|
|
Slit Lamp |
|
|
Ultrasound |
|
|
Computed tomography scan |
|
|
Laboratory |
|
|
RAPD: relative afferent pupillary defect; IOP: intraocular pressure |
|
Table 3. Summary of Severe Vision-Threatening Injuries |
|||
|
Condition |
Diagnostic |
ED Treatment |
Follow-up |
|
Retrobulbar hematoma and orbital compartment syndrome |
|
|
ED consult |
|
Orbital wall fracture |
|
|
Ophthalmology referral in 1-2 days |
|
Traumatic optic neuropathy |
|
|
ED consult |
|
Orbital apex syndrome |
|
|
ED consult |
|
Traumatic retinal detachments |
|
|
ED consult |
|
Commotio retinae |
|
|
Ophthalmology follow-up |
|
ED: emergency department; IOP: intraocular pressure; CT: computed tomography; IV: intravenous; PO: orally; ED: emergency department; EOM: extraocular movements; APD: afferent pupil defect |
|||
Differential Diagnosis: Severe Vision Threats
Retrobulbar Hemorrhage and Orbital Compartment Syndrome
A retrobulbar hemorrhage (RBH) describes bleeding posterior to the globe, usually as a result of blunt ocular trauma. The orbital compartment is limited in space and, thus, any bleeding within that closed space has serious implications. As the bleeding progresses, the enlarging hematoma displaces the globe anteriorly. This forward movement is limited by the globe being tethered posteriorly by the nerve sheath. This leads to a stretching of the optic nerve and blood vessels, resulting in venous compression. The compression compromises venous outflow and increases intraocular pressure (IOP). As pressures continue to increase, the central retinal artery is occluded, which then ultimately results in ischemic optic neuropathy. The end result is permanent vision loss.17 Increased pressure within the orbit causing end-organ damage is also called orbital compartment syndrome (OCS). It is estimated that the volume of the human orbit is about 30 mL. Even 7 mL of fluid has been shown to increase IOP.18 Irreversible damage to the optic nerve and retina occurs after 90-120 minutes of increased IOPs.17,19
RBH is relatively rare, with incidence rates from 0.088% to 3.6% in published studies of patients with facial trauma.20 The elderly and those using anticoagulants are at increased risk for RBHs after trauma.21 About 1% of RBH cases lead to OCS, so while RBH and OCS have overall low incidence rates, the morbidity from OCS is high, accounting for about half of all cases of traumatic blindness. 17,18 Therefore, it is important for the emergency clinician to be well-versed in the diagnosis and acute treatment of OCS.
The most common findings of an RBH are pain, proptosis, periorbital edema, subconjunctival ecchymosis, ophthalmoplegia, and mydriasis. If bleeding continues, compression of the retinal veins and arteries increases IOP and leads to an ischemic retina with a ‘‘cherry-red’’ macula, edematous and pale optic disc, and decreased visual
acuity. 17,20, 22-24
Not all these symptoms will be evident early in the disease process. As bleeding continues and pressures increase, there is progression and worsening of visual symptoms. Blindness and pupillary disturbances at initial presentation were most predictive of permanent blindness.17 The oculocardiac reflex also has been described in patients with RBHs. The stretch of the ocular muscles and increased IOP will activate the oculocardiac reflex, leading to a relative bradycardia.25
During the ocular exam in patients with suspected RBHs and OCS, pay particular attention to visual acuity, extraocular movements, proptosis, resistance to retropulsion, optic nerve edema, pupillary defects, and IOP measurement. Pupillary defects and IOP measurements become especially important in altered, intubated, or polytrauma patients.26
Bedside ultrasonography is another fast and reliable tool for assessing these patients. With ultrasonography, an RBH will appear as an anechoic structure in the typically hyperechoic retrobulbar area. In one study, emergency physicians performing bedside ultrasonography to diagnose RBH had a sensitivity of 99.7% and a specificity of 95.7%.27 Ultrasonography should be avoided if an obvious globe rupture is present.24 However, if careful measures are taken to apply as little pressure as possible, RBHs can be visualized with ultrasonography when a globe rupture is not obviously present. Lastly, a noncontrast, thin-slice computed tomography (CT) scan of the orbits is the imaging modality of choice for RBH.28
It must be emphasized that patients with findings of RBH during the examination or imaging might not always have OCS, and not every patient with an RBH needs a canthotomy and cantholysis. The best indication for decompression is an IOP of more than 40 mmHg in the setting of an RBH.19 A study by Erickson and Garcia sought to create a clinical decision-making tool to rule in OCS when IOP measurement is not available. The combination of relative proptosis, eyelids that are difficult to open with finger pressure, and the presence of an RAPD most likely indicate the need for an emergent decompression.29
OCS is a clinical diagnosis. Once it is diagnosed, the American Academy of Ophthalmology recommends a lateral canthotomy and cantholysis without waiting for further testing or imaging.22 Lateral canthotomy is discussed later.
Medical management, in addition to cantholysis, is suggested for treating patients with OCS. Mannitol 20% solution, 1.5 to 2 g/kg intravenous (IV) over 30 minutes and acetazolamide 500 mg IV or orally can both reduce IOP.30 Hydrocortisone, 100 mg IV, also can be considered to decrease muscle spasms and decrease edema.17, 31-33 Analgesics and antiemetics also should be administered to the patient.
However, medical management must never delay the definitive treatment for OCS, which is canthotomy and cantholysis. It is well demonstrated that cantholysis effectively reduces IOPs and, therefore, emergency clinicians must know how to perform the procedure.18 Patients with longer delays to cantholysis have more long-term complications, such as vision loss or blindness.17 Concurrently, while medically and procedurally managing a patient with OCS, ophthalmology should be consulted emergently.
Lateral Canthotomy in OCS. A lateral canthotomy is a relatively simple procedure. First, a local anesthetic, such as 2% lidocaine with epinephrine, is injected at the lateral canthus to provide analgesia. Then a hemostat is used to crush the lateral canthus. This helps with hemostasis. Next, an approximately 1 cm cut is made with a sterile scissor, extending laterally from the lateral lid angle to the lateral canthal ligament.
Some more blunt dissection might be needed to visualize the superior and inferior crus of the lateral canthal ligament. Often, the ligament will be easier to feel than to fully visualize; it often is described as feeling a taut guitar string. At this point, the inferior crus of the lateral canthal ligament can be cut until the lower lid loosens and the globe is able to displace itself anteriorly. Repeat testing of IOPs will show an immediate drop in pressures if the procedure is done correctly. The goal is to reduce the intraocular pressure to less than 40 mmHg. If this is not achieved, perform a cantholysis of the superior canthal ligament as well.25,34
Orbital Wall Fractures
Orbital fractures often are complex. The orbit is composed of multiple bones with various adjacent sinuses. Also, there are many nerves and muscles traversing this small area. The frontal, maxillary, ethmoidal, sphenoid, zygomatic, and lacrimal bones comprise the orbit. The maxilla and part of the zygomatic bone make up the orbital floor, with the maxillary sinus just inferior to it. The inferior rectus muscle and the maxillary branch of the trigeminal branch run closely along the orbital floor. The maxillary, lacrimal, and ethmoid bones make up the thin medial wall. The medial rectus muscle lies close to the medial wall. The zygomatic bone makes up the lateral wall while the frontal bone makes up the superior border.35-37
Orbital fractures are common in blunt ocular trauma, accounting for 4% to 16% of all facial fractures.37 Orbital fractures are seen more commonly in males and most often after falls, MVCs, or assaults.36 Isolated orbital fractures are referred to as blowout fractures, with orbital floor fractures being the most common.36,37
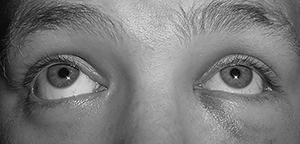
Specific exam findings that suggest an orbital fracture are highlighted in this section. First, patients may have an obvious bony step-off that can be palpated. With orbital floor fractures, findings consistent with infraorbital nerve damage can be seen, with either decreased sensation or hyperalgesia in the infraorbital region.35 More common than nerve injury are signs of entrapment of the inferior rectus muscle. Patients will have restriction in vertical movement of the affected eye. This presents as an upward gaze limitation or as diplopia with upward gaze. (See Figure 3.) Upward gaze also may be affected with entrapment of the periorbital fat or other periorbital soft tissue.37 Patients also may demonstrate bradycardia, nausea, or arrhythmias as part of the oculocardiac reflex.35
Figure 3. Impaired Upward Gaze From Blowout Fracture of Left Orbital Floor |
 |
|
Image courtesy of: J. Stephan Stapczynski, MD |
Pediatric patients may demonstrate “trapdoor” fractures, where clinically they do not demonstrate signs of entrapment. In these cases, a forced duction test can be performed to assess for entrapment.35,37 This involves using forceps to hold the sclera to attempt to move the eye upward after first applying adequate topical anesthetic; if there is resistance, the inferior rectus muscle may be entrapped. A forced duction test also is useful in patients unable to cooperate with the exam, such as intubated or altered patients; however, this is mostly left up to the ophthalmology consultants.
Medial orbital wall fractures can present with subcutaneous emphysema because of damage to the ethmoidal cells. The clinician also may visualize cerebrospinal fluid (CSF) rhinorrhea or epistaxis.35
Lateral wall fractures and superior wall fractures also are seen with blunt ocular trauma. These present with fewer complications, given the lack of sinuses and, thus, less chance of entrapment; most patients simply will need outpatient referral to oral maxillofacial surgery (OMFS) or otolaryngology (ENT).
In all orbital wall fractures, there might be signs of enophthalmos. However, because of the accompanying lid edema, patients usually look like they have proptosis rather than enophthalmos, and the enophthalmos does not become evident until weeks after the initial injury.35
A CT scan should be performed on patients suspected of having an orbital fracture. Entrapment might be visualized as herniation of orbital fat or extraocular muscles into the sinuses.36 (See Figure 4.) Like all imaging studies, CT scans are not 100% sensitive. Therefore, radiological studies should always be paired with a detailed physical examination to confirm entrapment. Magnetic resonance imaging (MRI) is not commonly used in trauma settings and, thus, there are very few studies looking at the utility of MRIs to assess for entrapment.
Figure 4. CT of Left Orbital Floor Fracture with Fat Herniation into Maxillary Sinus |
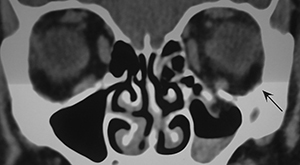 |
|
Image courtesy of: J. Stephan Stapczynski, MD |
Blowout fractures with a normal initial eye examination (visual acuity, pupil reactive, and ocular motility) can be managed as outpatients. Patients need a referral to ophthalmology within 24-48 hours for a repeat eye examination. Prophylactic oral antibiotics, usually 250-500 mg of cephalexin four times a day for 10 days, typically are started because of the belief that direct connection between the sterile orbit and the bacterial-colonized sinuses in most orbital fractures will lead to orbital cellulitis. However, this belief is without evidence to support its continued practice.38,39
Patients with orbital wall fractures can follow up with ophthalmology, plastic surgery, or OMFS for follow-up and delayed repair of the fracture if needed. Patients also should be instructed to sneeze with their mouths open and not to blow their nose. They should be instructed to return to the ED for any new or worsening symptoms.30 Any patients with signs of entrapment or displaying the oculocardiac reflex should receive an emergent ophthalmology consult.30
Traumatic Optic Neuropathy
The optic nerve can be subjected to injury in patients with significant facial trauma, both blunt and penetrating, resulting in traumatic optic neuropathy (TON). The diagnosis is clinical and supported by a history of severe, high-velocity facial trauma with an associated acute vision loss or deficit.
Patients can present with varied vision symptoms depending on the severity and location of the damaged optic nerve. Symptoms include varying degrees of vision loss, visual field deficits, dyschromatopsia, and a relative afferent pupil defect (RAPD). (See Figure 5.) This diagnosis can be challenging, especially in the acute polytrauma patient with associated head injury, who often is unable to participate in the exam.
Figure 5. Relative Afferent Pupil Defect |
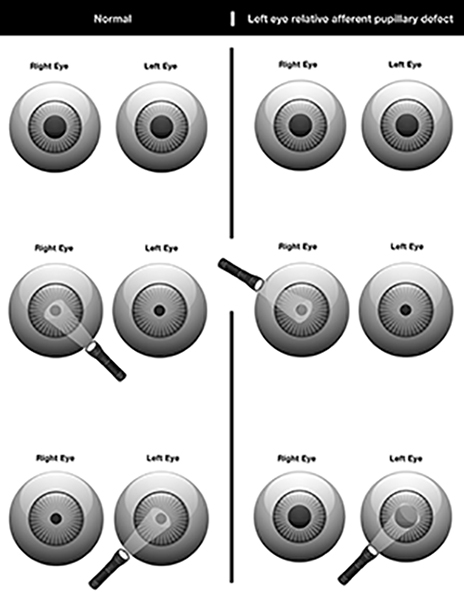 |
|
Image courtesy of: Tionne Myles-Smith. |
Head and facial CT imaging should be obtained to exclude any associated fractures or hemorrhage that could account for the TON and to evaluate for any associated injuries. Treatment largely is directed at the underlying cause of the injury (i.e., decompression for OCS).
Vision loss can be profound and permanent in these patients; however, there is a high rate of recovery over three to six months with supportive care and conservative management.40 Historically, optic nerve sheath decompression and steroids have been used as therapies for TON, but the largest studies have found no benefit for these two interventions.41 No treatment has been found to be more effective than observation. These patients warrant emergent ophthalmology consult after initial trauma stabilization.
Orbital Apex Syndrome
Orbital apex syndrome results from trauma around the optic nerve foramen and the superior orbital fissure. Patients present with visual loss (due to cranial nerve 2 injury) and ophthalmoplegia (due to cranial nerve 3, 4, and 6 injuries) from compression at the orbital apex. Orbital apex syndrome is a rare complication of maxillofacial trauma.42 If suspected, an emergent ophthalmology consultation must be obtained for guidance with imaging and management, which often is surgical.
Traumatic Retinal Detachments
Retinal detachments are relatively common, with an incidence of about 0.1 per 1,000 people annually. Traumatic detachments account for 10% to 20% of that total. Early recognition and management are imperative, since failure to identify and treat them in a timely manner can lead to permanent vision loss.
Retinal detachments traditionally are classified into three categories: rhegmatogenous, exudative, or tractional. Rhegmatogenous is the most common type of detachment, and more than 90% of retinal detachments secondary to trauma are rhegmatogenous in nature. The word is derived from the Greek word rhegma meaning “rupture” or “break.” The initial extent of vision loss is dependent on the size of the tear(s), the degree of separation, and whether there is macular involvement. Macular involvement has the most significant contribution to vision loss.
Rhegmatogenous retinal detachments (RRD) should be considered in any patient who presents to the ED with a history of blunt orbital trauma and sudden vision loss. Specifically, sports injuries in baseball, softball, basketball, boxing, and paintball-related injuries have some of the highest rates of traumatic RRD.43 There also is a significantly higher incidence in males and those with myopia.44
Presenting symptoms include photopsia, often described by patients as flashes of light. These occur as vitreoretinal traction develops. Floaters are another common presenting symptom and are caused by vitreous and cellular debris. RRDs typically begin in the periphery. As the detachment progresses more centrally, it often is described as a dark curtain or veil obscuring the central visual field. Once the macula is involved, there is severe or complete visual loss.45
In the vast majority of RRD cases, the external eye examination will be normal. The one exception is pupillary reflex testing. When at least 25% of the retina detaches, a partial APD can be detected on examination.46
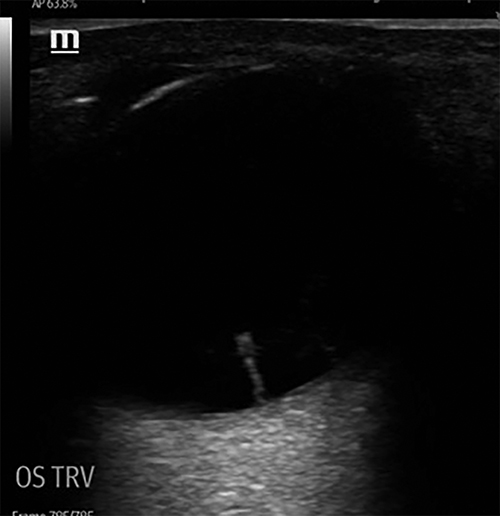
Thus, to diagnose an RRD, the retina must be visualized. While this can be done with fundoscopy, the mainstay in the ED has become diagnosis by ocular ultrasonography. Numerous studies have shown ultrasound to be an accurate tool for RRD diagnosis.47 RRD is visualized as a hyperechoic cord tethered to the posterior eye wall that moves like a sail with eye movements. (See Figure 6.) This should not be confused with posterior vitreous detachment, which also appears as a cord-like structure but with its movement not dependent on eye movement.48
Figure 6. Ultrasound Displaying a Hyperechoic Cord Tethered to Posterior Eye Wall |
|
Ultrasound displaying a hyperechoic cord tethered to the posterior eye wall that moves like a sail with eye movements. |
|
Image courtesy of: J. Stephan Stapczynski, MD |
Management in the ED setting includes an emergent ophthalmology consultation. Several surgical treatment options are available, and selection is dependent on considerations such as the patient’s age, comorbidities, other injuries, and extent of detachment (including macular involvement).
For the emergency clinician, one of the biggest potential complications is failure to diagnose. As mentioned before, vision might be preserved or the loss might be minimal until the detachment progresses to macular involvement. Once the macula is involved, studies have shown the extent of permanent visual loss increases with every day repair is delayed.49,50
Patients with blunt ocular trauma and a normal exam should be routinely referred to ophthalmology to ensure there are no underlying retinal breaks or posterior vitreous detachments, since both can progress to a retinal detachment. Patients also should be given strict return parameters for symptoms such as photopsia, floaters, and, of course, any new vision changes.
Commotio Retinae
Commotio retinae is a retinal injury that can be seen in blunt ocular trauma. The term was first described in 1873 by the German ophthalmologist Dr. Rudolf Berlin and, thus, is sometimes referred to as Berlin’s edema. Commotio retinae is caused by a contrecoup-type mechanism in which shockwaves originating from the site of impact traverse across the eye, damaging the external layer of photoreceptor cells and the retinal pigment epithelium opposite to the site of impact.51,52
Commotio retinae should be considered in any patient who presents to the ED with blunt ocular trauma. While this injury can occur in isolation with direct ocular trauma, it is not surprising that it also is seen in association with fractures of the orbitozygomaticomaxillary complex.53
These patients present with visual loss that varies in severity and is dependent on both the size of area involved and whether there is macular involvement.44 A patient with mild commotio retinae isolated to the peripheral retina might have no visual disturbances, while patients with macular involvement can experience complete visual impairment. When conducting an eye examination, the focus should be on the visual acuity and the fundoscopic examination.
Ultimately, diagnosis of commotio retinae requires a dilated exam of the fundus. The retina will have a grayish-white appearance correlating to the area of damaged cells.44 The fundus reflex, which typically appears red as light reflects off the back of the eye, can appear white in patients with significant retinal involvement. A pseudo-cherry red spot, similar to that in central retinal artery occlusion, can be seen if there is foveal involvement. With commotio retinae, the red spot appearance is an illusion caused by the surrounding peripheral whitening.54
There is no definitive ED treatment for commotio retinae. Patients without macular involvement generally are expected to have spontaneous resolution of symptoms over one to four weeks. All patients with commotio retinae diagnosed in the ED require ophthalmology follow-up within one week. Ophthalmologists can use advanced imaging for classification and prognostic estimations of visual recovery.55
REFERENCES
- Qadi M, Scott A, Prescott C. Economic trends in eye-related hospitalizations (PO118). Presented at AAO 2015, the 119th annual meeting of the American Academy of Ophthalmology. Las Vegas; November 2015.
- Yu J, Chen Y, Miao J, et al. Doubles trouble — 85 cases of ocular trauma in badminton: Clinical features and prevention. Br J Sports Med 2020;54:23-26.
- Hoskin AK, Yardley AME, Hanman K, et al. Sports-related eye and adnexal injuries in the Western Australian paediatric population. Acta Ophthalmol 2016;94:e407-e410.
- Haavisto AK, Sahraravand A, Puska P, Leivo Tl. Toy gun eye injuries — eye protection needed Helsinki ocular trauma study. Acta Ophthalmol 2019;97:430-434.
- Nemet AY, Asalee L, Lang Y, et al. Ocular paintball injuries. Isr Med Assoc J 2016;18:27-31.
- Jolly R, Arjunan M, Theodorou M, Dahlmann-Noor AH. Eye injuries in children — incidence and outcomes: An observational study at a dedicated children’s eye casualty. Eur J Ophthalmol 2019;29:499-503.
- Fineman MS. Ocular paintball injuries. Curr Opin Ophthalmol 2001;12:186-190.
- Pieramici DJ, Sternberg P Jr, Aaberg TM Sr, et al. A system for classifying mechanical injuries of the eye (globe). The Ocular Trauma Classification Group. Am J Ophthalmol 1997;123:820-831.
- Kuhn F, Maisiak R, Mann L, et al. The Ocular Trauma Score (OTS). Ophthalmol Clin North Am 2002;15:163-165, vi.
- Kuhn F, Morris R, Witherspoon CD, Birmingham Eye Trauma Terminology (BETT): Terminology and classification of mechanical eye injuries. Ophthalmol Clin North Am 2002;15:139-143, v.
- Scott R. The Ocular Trauma Score. Community Eye Health 2015;28:44-45.
- Mohseni M, Blair K, Bragg BN. Blunt eye trauma. StatPearls StatPearls Publishing; 2020.
- Rowh AD, Ufberg JW, Chan TC, et al. Lateral canthotomy and cantholysis: Emergency management of orbital compartment syndrome. J Emerg Med 2015;48:325-330.
- McInnes AW, Burnstine MA. White-eyed medial wall orbital blowout fracture. Ophthalmic Plast Reconstr Surg 2010;26:44-46.
- Fox A, Janson B, Stiff H, et al. A multidisciplinary educational curriculum for the management of orbital compartment syndrome. Am J Emerg Med 2020;38:1278-1280.
- Knoop K, Trott A. Ophthalmologic procedures in the emergency department--Part III: Slit lamp use and foreign bodies. Acad Emerg Med 1995;2:24-30.
- Christie B, Block L, Ma Y, et al. Retrobulbar hematoma: A systematic review of factors related to outcomes. J Plast Reconstr Aesthet Surg 2018;71:155-161.
- Dixon JL, Beams OK, Levine BJ, et al. Visual outcomes after traumatic retrobulbar hemorrhage are not related to time or intraocular pressure. Am J Emerg Med 2020;38:2308-2312.
- Amer E, El-Rahman Abbas A. Ocular compartment syndrome and lateral canthotomy procedure. J Emerg Med 2019;56:294-297.
- Bailey LA, van Brummen AJ, Ghergherehchi LM, et al. Visual outcomes of patients with retrobulbar hemorrhage undergoing lateral canthotomy and cantholysis. Ophthalmic Plast Reconstr Surg 2019;35:586-589.
- Berg BI, Flury E, Thieringer FM, et al. Retrobulbar haematoma in the era of anticoagulants. Injury 2019;50:1641-1648.
- Kondoff M, Nassrallah G, Ross M, Deschênes J. Incidence and outcomes of retrobulbar hematoma diagnosed by computed tomography in cases of orbital fracture. Can J Ophthalmol 2019;54:606-610.
- Trento Gdos S, Moura LB, de Oliveira Gorla LF, et al. The importance of early diagnosis of retrobulbar hemorrhage. J Craniofac Surg 2016;27:e581-e583.
- Kniess CK, Fong TC, Reilly AJ, Laoteppitaks C. Early detection of traumatic retrobulbar hemorrhage using bedside ocular ultrasound. J Emerg Med 2015.;49:58-60.
- Fahling JM, McKenzie LK. Oculocardiac reflex as a result of intraorbital trauma. J Emerg Med 2017;52:557-558.
- Welman T, Shanmugarajah K, Sabah S, et al. Assessment of emergency department eye examinations in patients presenting with mid-face injury. J Emerg Med 2016;50:422-426.
- Ojaghihaghighi S, Lombardi KM, Davis S, et al. Diagnosis of traumatic eye injuries with point-of-care ocular ultrasonography in the emergency department. Ann Emerg Med 2019;74:365-371.
- Gerbino G, Ramieri GA, Nasi A. Diagnosis and treatment of retrobulbar haematomas following blunt orbital trauma: A description of eight cases. Int J Oral Maxillofac Surg 2005;34:127-31.
- Erickson BP, Garcia GA. Evidence-based algorithm for the management of acute traumatic retrobulbar haemorrhage. Br J Oral Maxillofac Surg 2020;58:1091-1096.
- Walker RA, Adhikari S. Eye emergencies. In: Tintinalli JE, Yealy DM, Meckler GD, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. McGraw-Hill; 2021:1523-1559.
- Ujam A, Perry M. Emergency management for orbital compartment syndrome-is decompression mandatory? Int J Oral Maxillofac Surg 2016;45:1435-1437.
- Chen YA, Singhal D, Chen YR, et al. Management of acute traumatic retrobulbar haematomas: A 10-year retrospective review. J Plast Reconstr Aesthet Surg 2012;65:1325-1330.
- Winterton JV, Patel K, Mizen KD. Review of management options for a retrobulbar hemorrhage. J Oral Maxillofac Surg 2007;65:296-299.
- Lipke KJ, Gumbel HO. Emergency treatment of ocular trauma. Facial Plast Surg 2015;31:345-350.
- Joseph JM, Glavas IP. Orbital fractures: A review. Clin Ophthalmol 2011;5:95-100.
- Koenen L, Waseem M. Orbital floor fracture. StatPearls StatPearls Publishing; 2020.
- Kim HS, Jeong EC. Orbital floor fracture. Arch Craniofac Surg 2016;17:111-118.
- Pessino K, Cook T, Layliev J, et al. Excluding antibiotics in the management of nonoperative orbital and zygomatic fractures. Ann Plast Surg 2021;86:424-427.
- Esce AR, Chavarri VM, Joshi AB, Meiklejohn DA. Evaluation of antibiotic prophylaxis for acute nonoperative orbital fractures. Ophthalmic Plast Reconstr Surg 2021;Jan 19. doi: 10.1097/IOP.0000000000001915 [Online ahead of print].
- McClenaghan FC, Ezra DG, Holmes SB. Mechanisms and management of vision loss following orbital and facial trauma. Curr Opin Ophthalmol 2011;22:426-431.
- Chaon BC, Lee MS. Is there treatment for traumatic optic neuropathy? Curr Opin Ophthalmol 2015;26:445-449.
- Peter NM, Pearson AR. Orbital apex syndrome from blunt ocular trauma. Orbit 2010;29:42-44.
- Pieramici DJ. Sports-related eye injuries. JAMA 2017;318:2483-2484.
- Kuhn F, Pieramici DJ, eds. Ocular Trauma: Principles and Practice. Thieme; 2002.
- Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. Am Fam Physician 2004;69:1691-1698.
- Belliveau AP, Somani AN, Dossani RH. Pupillary light reflex. StatPearls StatPearls Publishing; 2021.
- Gottlieb M, Holladay D, Peksa GD. Point-of-care ocular ultrasound for the diagnosis of retinal detachment: A systematic review and meta-analysis. Acad Emerg Med 2019;26:931-939.
- Riguzzi C, Frenkel O, Nagdev A. Ocular ultrasound: Retinal detachment and posterior vitreous detachment. Academic Life in Emergency Medicine. Published March 11, 2014. http://www.aliem.com/ocular-ultrasound-retinal-detachment-posterior-vitreous-detachment/
- Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc 1982;80:475-497.
- Ross WH. Visual recovery after macula-off retinal detachment. Eye (Lond) 2002;16:440-446.
- Sipperley JO, Quigley HA, Gass DM. Traumatic retinopathy in primates. The explanation of commotio retinae. Arch Ophthalmol 1978;96:2267-2273.
- Li D, Akiyama H, Kishi S. Optical coherence tomography patterns and outcomes of contusion maculopathy caused by impact of sporting equipment. BMC Ophthalmol 2018;18:174.
- Johnson NR, Singh NR, Oztel M, et al. Ophthalmological injuries associated with fractures of the orbitozygomaticomaxillary complex. Br J Oral Maxillofac Surg 2018;56:221-226.
- Tripathy K, Patel BC. Cherry red spot. StatPearls StatPearls Publishing; 2020.
- Ahn SJ, Woo SJ, Kim KE, et al. Optical coherence tomography morphologic grading of macular commotio retinae and its association with anatomic and visual outcomes. Am J Ophthalmol 2013;156:994-1001 e1.
This article will focus on nonpenetrating eye injuries that are severe threats to vision.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.