Stroke Prevention in Nonvalvular Atrial Fibrillation: A Review of the Past, Present, and Future
April 1, 2023
Reprints
AUTHORS
Swapnil Garg, MD, Kettering Health Main Campus, Wright State University, Kettering, OH
Raja Nazir, MD, Kettering Health Main Campus, Wright State University, Kettering, OH
Nathaniel Dittoe, MD, Kettering Health Main Campus, Wright State University, Kettering, OH
PEER REVIEWER
Hunter Mwansa, MBBS, Frankel Cardiovascular Center, University of Michigan, Ann Arbor
EXECUTIVE SUMMARY
Atrial fibrillation is a very common cardiac disorder affecting 3 million to 6 million Americans and leading to varying degrees of symptoms and complications. Primary care physicians often are involved in the management and long-term care regarding antithrombotic therapy to prevent stroke and other embolic phenomena.
- Traditional pharmacological options include heparin, low molecular weight heparins, warfarin, aspirin, and clopidogrel.
- Direct oral anticoagulants have been shown to be noninferior or superior to warfarin for stroke prevention in nonvalvular atrial fibrillation. Surgical methods of appendage exclusion and excision have been met with varying degrees of success. Percutaneous left atrial occlusion devices represent the next phase of stroke prevention.
- Regarding stroke prevention, the last 15 years have seen extraordinary breakthroughs.
- The direct antithrombin anticoagulants and factor Xa inhibitors have predictable pharmacokinetics, fewer drug interactions than warfarin, no need for frequent monitoring, and are in many ways superior and safer. Chronic medication management results in continuing cost and risk of bleeding. Surgical options may mitigate the ongoing risk of bleeding and provide effective reduction in embolic events.
- The risks and benefits of procedural interventions are discussed, including surgical left atrial appendage ligation and percutaneous left atrial appendage closure.
Nonvalvular atrial fibrillation is a highly prevalent cardiac arrhythmia in the United States and often can be complicated by a thromboembolic phenomenon, the most concerning of which is stroke. In previous decades, vitamin K antagonists such as warfarin have been used for stroke prevention; however, the advent of various direct oral anticoagulants (DOACs) and left atrial appendage (LAA) exclusion methods have modified how healthcare providers treat patients with this common arrhythmia.
Direct oral anticoagulants have been studied extensively and have been shown to be noninferior or superior to warfarin for stroke prevention in nonvalvular atrial fibrillation (AF). Various surgical methods of appendage exclusion and excision have been met with varying degrees of long-term success. Percutaneous left atrial occlusion devices represent the next phase of stroke prevention in AF. This article reviews the current evidence for the use of various anticoagulants, surgical techniques, and the LAA occlusion devices currently available for stroke prevention in AF.
Introduction
AF is the most common cardiac arrhythmia in the United States. It is estimated that at least 3 million to 6 million Americans have AF.1 It can be insidious, and presentations can range from completely asymptomatic to extremely symptomatic with life-threatening complications and conditions such as congestive heart failure.2,3
Perhaps the most feared complication in AF is stroke. For decades, only vitamin K antagonists such as warfarin were known to be effective in stroke prevention.4 Over the last 15 years, there have been extraordinary breakthroughs in the prevention of stroke in AF. DOACs have revolutionized the approach to anticoagulation and stroke prevention. These agents have predictable pharmacokinetics, fewer drug interactions than warfarin, no need for frequent monitoring, and, in many ways, are superior in safety and stroke prevention.5
In the last decade, the rise of procedures to ligate or occlude the LAA for stroke prevention in AF is becoming mainstream. The LAA is responsible for the formation of approximately 90% of the thrombi that cause stroke in AF.6 The prospect of preventing stroke without the risks of anticoagulation are attractive, especially in patients who cannot tolerate anticoagulation or are at high risk of bleeding.
What previously was a binary choice of anticoagulation or no anticoagulation in the high bleeding risk population has expanded into a multitude of options, each with its own benefits and risks.
For the purpose of this review, we will focus on stroke prevention in nonvalvular AF, of which the definition itself is up for debate.7 In this review, the term nonvalvular AF refers to a patient with AF without hemodynamically significant mitral stenosis.
Lastly, and perhaps most importantly, considerations such as coexisting comorbidities, drug costs, and patient compliance always will be part of the decision-making process. These factors should help guide the provider and patient to the stroke prevention method that is best suited in each individual case.
Anticoagulants and Antiplatelet Agents
Warfarin, Aspirin, and Clopidogrel
Early trials established warfarin as a safe and effective therapy for stroke prevention in the setting of nonvalvular AF compared to aspirin and placebo. The SPINAF trial showed low-intensity warfarin (international normalized ratio [INR], 1.4 to 2.8) compared to placebo prevented cerebral infarction in patients with nonrheumatic AF with a risk reduction of 0.79 (95% confidence interval [CI], 0.52 to 0.90; P = 0.001).8 The AFASAK study compared warfarin to aspirin and placebo and showed a significantly lower incidence of thromboembolic complications and vascular mortality in the warfarin group than in the aspirin group and placebo group, which did not differ significantly.9
The SPAF-I trial compared 325 mg/day aspirin or warfarin with placebo and showed a primary endpoint (ischemic stroke and systemic embolism) reduction of 42% (P = 0.02) in the aspirin group and 67% (P = 0.01) in the warfarin group compared to placebo. Primary endpoint events or death were reduced 32% by aspirin (P = 0.02) and 58% by warfarin (P = 0.01) and all groups had similar bleeding rates.10
The SPAF-II trial compared warfarin to aspirin and assessed the rate of ischemic stroke and systemic embolism according to age. In patients younger than age 75 years, the primary event rate was 1.3% per year with warfarin compared to 1.9% per year with aspirin (relative risk [RR], 0.67; P = 0.24). In patients older than age 75 years, the primary event rate was 3.6% per year with warfarin compared to 4.6% per year with aspirin (RR, 0.73; P = 0.39). The results favored the use of warfarin over aspirin for all age groups.11
Since aspirin had been established to have a protective effect against thrombotic phenomena in AF, it was reasonable to expect that the addition of clopidogrel would have additional benefit. The ACTIVE W trial compared the combination of aspirin and clopidogrel to warfarin for prevention of vascular events: occurrence of stroke, non-central nervous system (CNS) systemic embolus, myocardial infarction, or vascular death. The annual risk of vascular events was 3.93% vs. 5.60% in the warfarin arm compared to the aspirin plus clopidogrel arm (RR, 1.44 [1.18 to 1.76; P = 0.0003]). The study was terminated early because of clear evidence of the superiority of oral anticoagulation therapy.12
Direct Thrombin Inhibitors
Dabigatran, a direct thrombin inhibitor, was the first DOAC approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of stroke and systemic thromboembolism as an alternative to warfarin in the setting of nonvalvular AF.13 The RE–LY trial compared two fixed doses (110 mg or 150 mg twice daily) of dabigatran against warfarin with a primary endpoint of stroke or systemic embolism. The primary endpoint rate was 1.69% per year in the warfarin group compared to 1.53% per year in the group receiving 110 mg of dabigatran (RR with dabigatran, 0.91; 95% CI, 0.74 to 1.11; P < 0.001 for noninferiority) and 1.11% per year in the group receiving 150 of mg dabigatran (relative risk, 0.66; 95% CI, 0.53 to 0.82; P < 0.001 for superiority). The rate of hemorrhagic stroke was significantly lower in both dabigatran dose groups compared to the warfarin group. The rate of major bleeding was significantly lower in the group receiving 110 mg of dabigatran but not in the group receiving 150 mg. Rates of life-threatening bleeding and intracranial bleeding were significantly higher in the warfarin group than with either dose in the dabigatran groups. Major gastrointestinal bleeding was statistically higher in the dabigatran 150 mg group compared to the warfarin group.14
Factor Xa Inhibitors
Rivaroxaban, a direct factor Xa inhibitor, was the second DOAC approved by the FDA for stroke risk reduction in the setting of nonvalvular AF. Predominantly renally excreted, it should be given as a single daily dose with the evening meal for adequate absorption.15
The ROCKET AF trial was a noninferiority study comparing rivaroxaban to warfarin for stroke or systemic embolism prevention in patients with nonvalvular AF. In the per-protocol analysis, the primary endpoint occurred at a rate of 1.7% per year in the rivaroxaban group vs. 2.2% per year in the warfarin group (hazard ratio [HR] in the rivaroxaban group, 0.79; 95% CI, 0.66 to 0.96; P < 0.001 for noninferiority). In the intention-to-treat analysis, superiority was not achieved (P = 0.12). Rates of major bleeding were similar between the two groups. Rates of intracranial hemorrhage and fatal bleeding were significantly lower in the rivaroxaban group than in the warfarin group, but major gastrointestinal bleeding was more common in the rivaroxaban group.13
Apixaban, another factor Xa inhibitor, was the third DOAC approved by the FDA for stroke risk reduction in the setting of nonvalvular AF. Predominantly hepatically cleared, it has been evaluated in two major clinical trials.13
In the ARISTOTLE trial, apixaban (5 mg twice daily) was compared with warfarin (target INR of 2 to 3) for noninferiority with a primary outcome of ischemic or hemorrhagic stroke. The primary outcome occurred at 1.27% per year in the apixaban group compared to 1.60% per year in the warfarin group (HR with apixaban, 0.79; 95% CI, 0.66 to 0.95; P < 0.001 for noninferiority; P = 0.01 for superiority). There was less major bleeding, a lower death rate, and less hemorrhagic stroke in the apixaban group.16
In the AVERROES study, apixaban was compared to aspirin in patients who were not suitable candidates for warfarin. The trial was terminated early because of the clear benefit of apixaban over aspirin. The rate of primary outcome in the apixaban group was 1.6% per year and 3.7% per year in the aspirin group (HR with apixaban, 0.45; 95% CI, 0.32 to 0.62; P < 0.001).17
Edoxaban, a factor Xa inhibitor, was compared to warfarin in the ENGAGE AF-TIMI-48 trial for the prevention of stroke and systemic embolism. The annualized primary endpoint rate during treatment was 1.50% with warfarin compared to 1.18% with high-dose (60 mg daily) edoxaban (HR, 0.79; 97.5% CI, 0.63 to 0.99; P < 0.001 for
noninferiority). For low-dose (30 mg daily) edoxaban, the annualized rate of primary endpoint was 1.61% (HR, 1.07; 97.5% CI, 0.87 to 1.31; P = 0.005 for noninferiority). Both doses of edoxaban were noninferior to warfarin and were associated with lower rates of bleeding and death from cardiovascular causes.18
Since the drug is 50% renally cleared, there has been some concern for relatively lower efficacy for the prevention of stroke or systemic embolism with the lower dose regimen of edoxaban compared to warfarin in patients with a creatinine clearance (CrCl) > 95 mL/min. Because of the lower rates of major bleeding, the net clinical benefit was more favorable with a higher dose regimen across the range of CrCl.18 Additionally, in patients with CrCl > 95 mL/min, low-dose edoxaban had less reduction of stroke and systemic embolism when compared to warfarin. This illustrates the importance of using high-dose edoxaban in patients with CrCL > 95 mL/min.19
Fondaparinux, a selective factor Xa inhibitor administered by subcutaneous injection, is renally excreted and carries the advantage of once-daily dosing without a requirement for therapeutic level monitoring. It has been shown to be beneficial in the treatment of venous thromboembolism and acute coronary syndrome.20,21
The SAFE-AF trial compared fondaparinux to unfractionated heparin plus vitamin K antagonist for the prevention of thromboembolic complications, death, and major bleeding events in patients undergoing cardioversion in nonvalvular AF. The primary endpoint occurred in 1.7% patients in the fondaparinux group and 1.2% in the unfractionated heparin plus vitamin K antagonist group. The rate of thrombus disappearance among clot-positive patients was higher in the fondaparinux arm, with similar adverse events between the two groups.22
Heparins and Low Molecular Weight Heparins
Heparin and its derivative, low molecular weight heparin (LMWH), are useful when rapid anticoagulation is required because of rapid onset of action when given parenterally. Intravenous (IV) heparin therapy generally is limited to the hospital setting, where its therapeutic effect can be monitored frequently.
In contrast, LMWH preparations can be given in or outside of hospital settings because of subcutaneous administration without monitoring requirements. These preparations originally were used for treatment of thrombosis and coronary artery disease.23
Current AF guidelines give a class IIa recommendation for the administration of heparin, a factor Xa inhibitor, or a direct thrombin inhibitor for patients with AF or atrial flutter with a CHA2DS2-VASc score of 2 or greater in men and 3 or greater in women of less than 48 hours as soon as possible prior to cardioversion, followed by long-term anticoagulation therapy.24 In the BRIDGE trial, researchers studied whether patients who were anticoagulated on warfarin for AF and were undergoing elective operations or procedures would benefit from bridging anticoagulation. The trial showed that forgoing bridging anticoagulation was noninferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism and decreased the risk of major bleeding.25
Surgical Left Atrial Appendage Ligation
Surgical occlusion of the LAA is thought to reduce the risk of thromboembolic events in patients with AF. However, the procedure remains controversial for several reasons, and, thus, remains a class IIb recommendation with B-NR level of evidence in patients undergoing cardiac surgery.12 Removal of the LAA yields inconsistent results, partially because the anatomy of the LAA is quite variable.
One magnetic resonance imaging (MRI) study evaluated patients with AF and showed significant variability in the dimensions and morphology of the LAA.26 Additionally, the nearby vital cardiac structures present a technical challenge when surgically closing the os of the LAA. The LAA lies in close proximity to the left superior pulmonary vein posteriorly and the mitral valve annulus anteriorly. The left anterior descending coronary artery and left circumflex coronary artery run close to the LAA epicardially. Clinical studies have not shown evidence of disruption to the pulmonary vein inflow or mitral valve function; however, the left anterior descending coronary artery and the left circumflex coronary artery can be vulnerable to trauma.27
A variety of surgical techniques for left atrial occlusion have been used and, broadly, can be divided into two categories: exclusion and excision. With exclusion methods, the ligation can occur on the epicardial surface or from the endocardial surface.
As for excision methods, the most common technique is the stapled excision or the removal and oversew.28 The use of an automatic stapling device with a rotatable head has been shown to be safe and effective in a small study.29 Surgical closure of the LAA often is incomplete.
In one study, transesophageal echocardiogram was used to assess patients undergoing LAA closure at the time of mitral valve surgery for completeness of closure. The patients all underwent ligation from the endocardial surface using sutures and were found to have an incomplete occlusion incidence of 36%.30 In a subsequent larger analysis, only 40% of LAA closures were successful. Successful closures occurred more often with excision (73%) than suture exclusion (23%) and stapler exclusion (0%) (P < 0.001).31
Surgical closure of the LAA had been done for many years without randomized data to evaluate its safety or efficacy. This first was studied in the randomized controlled LAAOS trial. Patients undergoing coronary artery bypass grafting with risk factors for stroke were randomized to LAA occlusion or a control group. The LAA was closed using sutures or a stapling device. Postoperative transesophageal echocardiography (TEE) was performed to evaluate the completeness of LAA occlusion.
There was no significant difference between the two groups in terms of complications; however, completeness of occlusion was achieved in 45% of the cases using sutures and 72% using a stapler device (P = 0.14).32
Another single institutional trial randomized patients undergoing AF surgery prospectively into one of three LAA elimination techniques (internal suture ligation, external stapled excision, or surgical excision) and found an overall failure rate of 57%, with no significant difference between the three groups.33
The first atrial exclusion device was reported in 2005, which consisted of a rigid and flexible stainless steel member completely covered with a braided polyester knit sheath to promote ingrowth.
Initially, the device was tested in the canine model, and complete exclusion of the LAA from the circulation was confirmed.34 A second iteration of the device subsequently was developed, which consisted of two parallel, curved, rigid titanium tubes covered in urethane elastomer and a knot-braided polyester sheath and two nitinol springs.
This device also was tested in canines, with complete appendage exclusion, no complications, no device migration, and no damage to surrounding structures.35 Since then, the AtriClip device has been modified (AtriClip PRO V) and now is available in four sizes ranging from 35 mm to 50 mm.
Toale et al performed a systematic review and identified 922 patients who underwent AtriClip placement as part of another cardiac procedure or as a standalone procedure. LAA occlusion was achieved in 97.8% of patients, with no device-related adverse events; however, criteria for successful placement varied, most commonly as no residual flow to the LAA or a residual left atrial stump < 10 mm.36
One study used transesophageal echocardiography to measure the depth of the residual LAA pouch (defined as the distance from the ostial plane to the deepest part of the residuum) in 40 patients who underwent closure with the AtriClip Pro. The residual stump depth was found to be 12.9 mm ± 5.9 mm and there were no re-cannulated LAAs. Overall, the AtriClip device has been shown to be safe and efficacious, with the presence of a small residual pouch after occlusion, which has not been shown to be associated with a higher risk of stroke.37
Percutaneous Left Atrial Appendage Closure
Watchman Left Atrial Appendage Occlusion System
The Watchman LAA system consists of a nitinol cage with a polytetrafluoroethylene membrane on the surface and fixation barbs around the perimeter. The system also contains a transseptal access sheath and a delivery catheter. The Watchman implant is available in diameters of 21 mm, 24 mm, 27 mm, 30 mm, and 33 mm to accommodate multiple unique anatomies. The system is inserted into the femoral vein and delivered to the LAA via atrial transseptal puncture under TEE.38 (See Figures 1 and 2.)
Figure 1. 2D TEE Image of Deployed Watchman Device in Left Atrial Appendage |
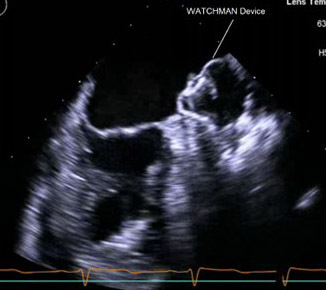 |
TEE: transesophageal echocardiography |
Figure 2. 3D “Top Down” TEE Image of Deployed Watchman Device in Left Atrial Appendage |
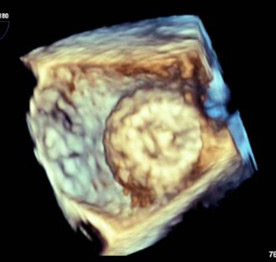 |
TEE: transesophageal echocardiography |
In an initial evaluation, 75 patients were enrolled and 66 underwent successful implantation of the device. Of the remaining nine patients, one had a right groin scar precluding transseptal sheath placement, one had a core wire malfunction, and seven had LAA anatomy unsuitable for device placement. The primary endpoint studied was device position at 45 days post-placement. Successful placement was measured by TEE as LAA completely sealed with the absence of or minimal flow around the device (jet of < 3 mm). Fifty-four of the 58 patients who came for 45-day follow-up were found to have successful placement of the device, showing the safety and feasibility of the Watchman system.39
The PROTECT AF trial examined the efficacy and safety of percutaneous closure of the LAA with the Watchman device and tested noninferiority of the device against warfarin therapy in patients with nonvalvular AF.39 Patients were randomized in a 2:1 ratio to device or control groups. A total of 707 patients were randomized, with 463 patients assigned to LAA closure and 244 patients assigned to the control group. At 45-day follow-up, 86% of patients with an implanted device met the TEE criteria for complete closure and were able to stop taking warfarin. The primary efficacy event rate was 3.0 per 100 patient-years (95% credible interval [CrI], 1.9 to 4.5) in the intervention group and 4.9 per 100 patient-years (2.8 to 7.1) in the control group (rate ratio, 0.62; 95% CrI, 0.35 to 1.25), thus meeting the prespecified criteria for noninferiority of the intervention.
Primary safety events, defined in the trial as any life-threatening bleeding, including pericardial effusion requiring drainage, intracranial bleeding, or gastrointestinal bleeding requiring transfusion, occurred more frequently in the intervention group than in the control group (7.4 per 100 patient-years; 95% CrI, 5.5 to 9.7 vs. 4.4 per 100 patient-years; 95% CrI, 2.5 to 6.7; RR, 1.69, 1.01 to 3.19).40
Further long-term data regarding the Watchman device were studied in the Continued Access Protocol (CAP), a nonrandomized registry beginning at the conclusion of the PROTECT AF trial. Because of the concern about higher primary safety events in the PROTECT AF trial, patients were enrolled in the CAP registry (n = 460). The safety endpoint included bleeding and procedure-related events (pericardial effusion, stroke, and device embolization).
There was a significant decline in the rate of procedure or device-related safety events within seven days of the procedure between the two studies. The rate of serious pericardial effusion within seven days of implantation was lower in the CAP registry as well. These results suggested there was a significant improvement in the safety of the Watchman device implantation with increased operator experience.41
The Watchman device was evaluated further in the PREVAIL study, which randomized patients with nonvalvular AF in a 2:1 fashion to receive the Watchman device or receive chronic warfarin therapy. At 18-month follow-up, the first co-primary efficacy endpoint (composite of stroke, systemic embolism, and cardiovascular/unexplained death) did not achieve prespecified criteria for noninferiority; however, the second co-primary efficacy endpoint (stroke or systemic embolization > 7 days post-randomization) did achieve noninferiority. Early safety events occurred in 2.2% of the Watchman arm, which was significantly lower than in PROTECT AF. Pericardial effusions requiring surgical repair decreased significantly, and those requiring pericardiocentesis also decreased, although not statistically significantly.42
Five-year outcome data of the PROTECT AF and PREVAIL studies demonstrated that LAA closure with the Watchman device provided comparable stroke prevention to warfarin with additional reductions in major bleeding and mortality.43
The Watchman FLX, the next-generation LAA closure device, subsequently was developed to further minimize the risk of pericardial effusion, device embolization, and periprocedural stroke. The device consists of a self-expanding nitinol frame with 18 peripheral fixation anchors (two rows of nine) and a permeable polyester fabric covering the atrial-facing surface. Compared to the previous device, the next-generation device covers a slightly larger range of LAA diameters and can accommodate a wider variety of anatomies. The 18-strut design compared to the 10-strut design allows the device to conform to the LAA ostium and reduce peri-device leak.
This device was studied in the PINNACLE FLX trial, which was a prospective, nonrandomized, multicenter study enrolling 400 patients. LAA closure with the Watchman FLX was found to have a high incidence of effective appendage closure and low incidence of safety events. Pericardial effusion is the most common procedure-related complication. In the National Cardiovascular Data Registry’s LAAO Registry of commercial implants within the United States, in-hospital pericardial effusion requiring surgical or percutaneous drainage occurred in 1.4%. In this study, there were no pericardial effusions requiring drainage in the first seven days.44
Amplatzer Amulet
The Amulet is the next generation of the Amplatzer cardiac plug and is a self-expanding device for LAA occlusion implanted from the femoral vein using a transseptal approach. (See Figures 3 and 4.) It consists of a distal lobe, which conforms to the inner LAA wall, connected by an articulated waist to a proximal disk, which seals the LAA ostium. In an initial evaluation, 24 out of 25 patients underwent successful device implantation without any procedural stroke, pericardial effusion, or device embolization. At follow-up, there was no residual leak > 3 mm or device embolization, demonstrating the safety and feasibility of the device.45
Figure 3. 2D TEE Image of Amulet Device Deployment into Left Atrial Appendage |
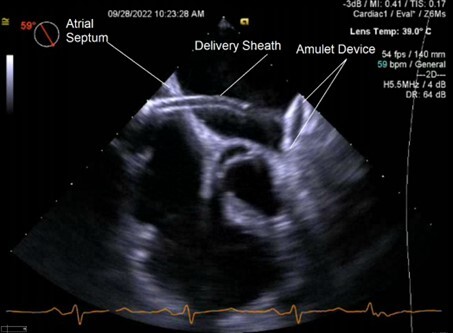 |
TEE: transesophageal echocardiography |
Figure 4. 3D “Top Down” TEE Image of Amulet Device in Left Atrial Appendage |
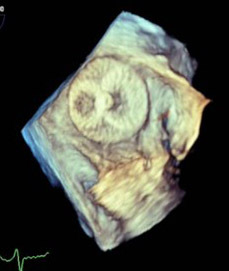 |
TEE: transesophageal echocardiography |
The Amplatzer Amulet prospective global observational study followed 1,088 patients with the device for two years and reported a 99.1% implant success rate, a peridevice flow < 3 mm in 98.4% at follow-up TEE, and an ischemic stroke rate of 2.2% per year — a 67% reduction compared to the CHA2DS2-VASc predicted rate. Major adverse events occurred in 4%, device-related thrombus was seen in 1.6%, and cardiovascular death or ischemic stroke occurred in 8.7% of patients at two years. Major bleeding occurred at 10.1% per year for the first year and at 4.0% per year for the second year.
The large number of first-year bleeds were attributed to a high-risk population for major bleeding as well as dual antiplatelet or anticoagulation therapy in a large number of patients at discharge. A large portion of first-year bleeds occurred prior to transition to aspirin monotherapy within the first three months. Additionally, periprocedural major bleeding contributed to 3.1% of the 10.1% first-year bleeding rate.46
The Amulet IDE trial compared the Amulet LAA occluder safety and effectiveness with the Watchman device. The primary safety endpoint was a composite of procedure-related complications, all-cause death, or major bleeding through 12 months. The primary effectiveness endpoint was a composite of ischemic stroke or systemic embolism through 18 months. The primary mechanism of action endpoint was successful device-based LAA occlusion, which was defined as residual jet around the device ≤ 5 mm.
The Amulet occluder was noninferior to the Watchman device for the primary safety endpoint (14.5% vs. 14.7%; difference = -0.14 [95% CI, -3.42 to 3.13]; P < 0.001 for noninferiority). Major bleeding and all-cause death were similar between the two groups, but procedure-related complications were higher for the Amulet occluder (4.5% vs. 2.5%). The Amulet occluder was noninferior to the Watchman device for the primary effectiveness endpoint. There were similar bleeding rates between the two groups. LAA occlusion was higher for the Amulet occluder than for the Watchman (98.9% vs. 96.8%; difference = 2.03 [95% CI, 0.41 to 3.66]; P < 0.001 for noninferiority; P = 0.003 for superiority).
The procedural complications were nearly twice as high with the Amulet occluder, driven mostly by pericardial effusion and device embolization. In general, the complications occurred early in an implanter’s experience. Patients discharged on anticoagulation therapy experienced a higher rate of late pericardial effusion compared to those discharged on no antithrombotic therapy.47
The SWISS-APERO trial was conducted to compare the Amulet LAA closure device to the next-generation Watchman FLX. The primary endpoint studied was the composite of justified crossover to the nonrandomly allocated device or 45-day LAA patency rate with coronary computed tomographic angiography (CCTA) and occurred in 67.6% of patients receiving the Amulet and 70.0% of patients receiving the Watchman (risk ratio, 0.97 [95% CI, 0.80 to 1.16]; P = 0.713). There were more major procedure-related complications in the Amulet group as the result of more frequent bleeding. At 45 days, the peridevice leak rate with TEE was higher with Watchman than with Amulet (27.5% vs. 13.7%, P = 0.020), but none were major (> 5 mm). The clinical relevance of CCTA-detected patency will require further study.47
The LARIAT
The LARIAT was granted 510k Class II clearance by the FDA for soft tissue approximation and has been used to ligate the LAA percutaneously. In addition to stroke prevention, it has been used as an antiarrhythmic strategy as well.48 The procedure involves pericardial and transseptal access followed by placement of an endocardial magnet-tipped guidewire in the apex of the LAA followed by balloon identification of the LAA os. The epicardial magnet-tipped guidewire then is connected to the endocardial guidewire for stabilization, followed by snare capture of the LAA.49 An initial canine study showed safe and reliable ligation of the entire LAA using a closed-chest approach with endothelialization of the LAA orifice.50
An initial clinical experience observational study enrolled 89 patients to undergo percutaneous ligation with the LARIAT. Eighty-five patients underwent successful LAA ligation, with 81 patients achieving complete immediate closure. Three patients had a ≤ 2 mm residual leak by TEE and one patient had a < 3 mm residual leak. Three patients experienced access-related complications. Sixty-five patients underwent one-year TEE and there was 98% complete LAA closure, including the patients with previous leaks. This study demonstrated the LARIAT device can be performed effectively with low access complications and periprocedural adverse events.51
Researchers studied a large registry data of 712 patients undergoing LARIAT at 18 U.S. hospitals for the primary endpoint of successful suture deployment, no leak by intraprocedural TEE, and no major complication at discharge. A 2 mm to 5 mm leak also was studied as a secondary endpoint. Successful deployment occurred in 682 patients (95.5%), with complete closure achieved in 669 patients (98%), and 13 patients (1.8%) had trace leak (< 2 mm). Ten patients had cardiac perforation requiring open-heart surgery, while another 14 patients did not need surgery. Introduction of the micropuncture needle pericardial access decreased the risk of cardiac perforation. Delayed complications, including pericarditis and pericardial/pleural effusion, occurred in 34 patients, which decreased significantly with use of periprocedural colchicine. A 2 mm to 5 mm leak was seen in 6.5% of patients.52
Although no randomized study has been done, a prospective observational study of patients undergoing successful Watchman and LARIAT device implantation showed a greater number of leaks in the Watchman group than in the LARIAT group (46 [21%] vs. 33 [14%]; P = 0.019), with larger leaks in the Watchman group as well (3.10 mm ± 1.5 mm vs. 2.15 mm ± 1.3 mm; P = 0.001). The Watchman group had one device embolization requiring surgery and two pericardial effusions requiring pericardiocentesis. The LARIAT group had four patients with cardiac tamponade requiring urgent surgical repair.53 The LARIAT, performed in conjunction with extensive ablation in patients with longstanding persistent AF, also may carry the benefit of freedom from atrial arrhythmias.54
The WaveCrest LAA closure device is a single lobe device consisting of a self-expanding nitinol frame covered by a polytetrafluoroethylene knit fabric with 20 peripherally located fixation hooks to anchor the device. The device is unique in that it has no radial force to aid with stability and the hooks are retractable.55 The device is currently pending FDA approval and is being compared against the Watchman device in the ongoing Wavecrest2 trial, with an estimated primary completion date in early 2024.56
At the time of publication, there are a myriad of other novel percutaneous LAA closure devices in various stages of clinical development. Future research may lead to the use of these devices in the coming years.57
Future Directions
Factor XI inhibitors have been identified in animals and, more recently, in epidemiological studies, as a potential target for anticoagulants with a better safety profile because factor XI deficiency protects against thrombosis and is associated with little or no bleeding. The concern for bleeding with DOACs has resulted in widespread under-prescribing in patients with AF. Factor XI inhibitors can attenuate thrombosis with little or no disruption of hemostasis. With this evidence, factor XI inhibitors have become a target for anticoagulant therapy, and several agents are in various stages of development.58 The PACIFIC-AF trial was a double-blinded Phase II dose-finding study in which the factor XIa inhibitor asundexian 20 mg and 50 mg once daily was found to have lower rates of bleeding compared with the standard dosing of apixaban with near-complete in-vivo factor XIa inhibition in patients with AF.59
With the advent of LAA occlusion devices, the current focus has been on the use of these devices as a second-line therapy in patients who have significant risks of anticoagulation or who have had bleeding complications. The question of using these devices as first-line treatment for stroke prevention in patients with lower risks of stroke and bleeding complications has been one of debate.60 The CHAMPION AF study is a prospective, randomized, multi-center global trial designed to evaluate the Watchman FLX device as first-line stroke prevention therapy as an alternative to DOACs. The study has finished enrollment, with an estimated completion date of December 2027.61 Similarly, the CATALYST trial is an ongoing randomized control trial involving 2,650 patients to study the use of the Amplatzer Amulet as an alternative to DOACs, with an estimated completion date of April 2029.62
Summary
Methods to prevent stroke in AF have evolved significantly over the past two decades. DOACs, LAA occlusion/exclusion devices, and surgical LAA closure techniques are effective therapies now. The future development of additional implantable devices and potentially safer, more effective anticoagulants is an exciting prospect. One might wonder if future generations of medical providers will look upon the days of using warfarin, a repurposed rat poison, with the same curiosity and amazement as we view the medicinal use of leeches in antiquity. Ironically, leeches have helped pioneer modern anticoagulants and have found a new modern medical use in microsurgery.63,64 Perhaps warfarin will persist as a niche anticoagulant or potentially be repurposed once again. Although it is impossible to tell what the future will hold, given the high prevalence of AF, it seems stroke prevention will continue to be an important aspect of AF treatment.1
References
- Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ Res 2020;127:4-20.
- Dilaveris PE, Kennedy HL. Silent atrial fibrillation: Epidemiology, diagnosis, and clinical impact. Clin Cardiol 2017;40:413-418.
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003;107:2920-2925.
- Waldo AL. Stroke prevention in atrial fibrillation. JAMA 2003;290:1093-1095.
- Lee JJ, Ha ACT, Dorian P, et al. Meta-analysis of safety and efficacy of direct oral anticoagulants versus warfarin according to time in therapeutic range in atrial fibrillation. Am J Cardiol 2021;140:62-68.
- Jackson LR 2nd, Jackson KP, Thomas KL. Percutaneous left atrial appendage occlusion: A review of current devices, clinical evidence, patient selection, and post procedural antithrombotic management. Prog Cardiovasc Dis 2021;66:92-100.
- Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis 2015;108:530-539.
- Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406-1412. Erratum in: N Engl J Med 1993;328:148.
- Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1989;1:175-179.
- [No authors listed]. Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation 1991;84:527-539.
- [No authors listed]. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687-691.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): A randomised controlled trial. Lancet 2006;367:1903-1912.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199-267. Erratum in: Circulation 2014;130:e272-274.
- Eikelboom JW, Connolly SJ. Explaining the RE-LY Trial. Can J Hosp Pharm 2010;63:334-336.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-891.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-992.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-817.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-2104.
- Yu HT, Yang PS, Kim TH, et al. Impact of renal function on outcomes with edoxaban in real-world patients with atrial fibrillation. Stroke 2018;49:2421-2429.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):381S-453S.
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-e228. Erratum in: J Am Coll Cardiol 2014;64:2713-2714.
- Cohen A, Stellbrink C, Le Heuzey JY, et al. SAfety of Fondaparinux in transoesophageal echocardiography-guided Electric cardioversion of Atrial Fibrillation (SAFE-AF) study: A pilot study. Arch Cardiovasc Dis 2015;108:122-131.
- Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001;119(1 Suppl):64S-94S.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-132. Erratum in: J Am Coll Cardiol 2019;74:599.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823-833.
- Heist EK, Refaat M, Danik SB, et al. Analysis of the left atrial appendage by magnetic resonance angiography in patients with atrial fibrillation. Heart Rhythm 2006;3:1313-1318.
- Su P, McCarthy KP, Ho SY. Occluding the left atrial appendage: Anatomical considerations. Heart 2008;94:1166-1170.
- Chatterjee S, Alexander JC, Pearson PJ, Feldman T. Left atrial appendage occlusion: Lessons learned from surgical and transcatheter experiences. Ann Thorac Surg 2011;92:2283-2292.
- DiSesa VJ, Tam S, Cohn LH. Ligation of the left atrial appendage using an automatic surgical stapler. Ann Thorac Surg 1988;46:652-653.
- Katz ES, Tsiamtsiouris T, Applebaum RM, et al. Surgical left atrial appendage ligation is frequently incomplete: A transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36:468-471.
- Kanderian AS, Gillinov AM, Pettersson GB, et al. Success of surgical left atrial appendage closure: Assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924-929.
- Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-293.
- Lee R, Vassallo P, Kruse J, et al. A randomized, prospective pilot comparison of 3 atrial appendage elimination techniques: Internal ligation, stapled excision, and surgical excision. J Thorac Cardiovasc Surg 2016;152:1075-1080.
- Kamohara K, Fukamachi K, Ootaki Y, et al. A novel device for left atrial appendage exclusion. J Thorac Cardiovasc Surg 2005;130:1639-1644.
- Kamohara K, Fukamachi K, Ootaki Y, et al. Evaluation of a novel device for left atrial appendage exclusion: The second-generation atrial exclusion device. J Thorac Cardiovasc Surg 2006;132:340-346.
- Toale C, Fitzmaurice GJ, Eaton D, et al. Outcomes of left atrial appendage occlusion using the AtriClip device: A systematic review. Interact Cardiovasc Thorac Surg 2019;29:655-662.
- Osmancik P, Budera P, Zdarska J, et al. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the AtriClip PRO device. Interact Cardiovasc Thorac Surg 2018;26:919-925.
- Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007;49:1490-1495.
- Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am Heart J 2006;151:956-961.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009;374:534-542.
- Reddy VY, Holmes D, Doshi SK, et al. Safety of percutaneous left atrial appendage closure: Results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011;123:417-424.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014;64:1-12.
- Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70:2964-2975.
- Kar S, Doshi SK, Sadhu A, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: Results from the PINNACLE FLX trial. Circulation 2021;143:1754-1762.
- Freixa X, Abualsaud A, Chan J, et al. Left atrial appendage occlusion: Initial experience with the Amplatzer™ Amulet™. Int J Cardiol 2014;174:492-496.
- Hildick-Smith D, Landmesser U, Camm AJ, et al. Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: Full results of the prospective global observational study. Eur Heart J 2020;41:2894-2901.
- Lakkireddy D, Thaler D, Ellis CR, et al. Amplatzer Amulet left atrial appendage occluder versus Watchman device for stroke prophylaxis (Amulet IDE): A randomized, controlled trial. Circulation 2021;144:1543-1552.
- Galea R, De Marco F, Meneveau N, et al. Amulet or Watchman device for percutaneous left atrial appendage closure: Primary results of the SWISS-APERO randomized clinical trial. Circulation 2022;145:724-738.
- Musat D, Mittal S. LARIAT trial updates. J Atr Fibrillation 2018;11:1806.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: Initial clinical experience. J Am Coll Cardiol 2013;62:108-118.
- Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: Initial experience in a canine model. Circ Cardiovasc Interv 2010;3:224-229.
- Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: Results from a US multicenter evaluation. Heart Rhythm 2016;13:1030-1036.
- Pillarisetti J, Reddy YM, Gunda S, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: Understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm 2015;12:1501-1507.
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol 2016;68:1929-1940.
- Gianni C, Della Rocca DG, Natale A, Horton RP. Interventional treatment for stroke prevention. Korean Circ J 2021;51:1-14.
- ClinicalTrials.gov. WAveCrest Vs. Watchman TranssEptal LAA Closure to REduce AF-Mediated STroke 2 (WAVECREST2). National Library of Medicine. Last updated Feb. 2, 2022. https://beta.clinicaltrials.gov/study/NCT03302494
- Cruz-González I, Trejo-Velasco B. Percutaneous left atrial appendage occlusion in the current practice. Kardiol Pol 2021;79:255-268.
- Fredenburgh JC, Weitz JI. Factor XI as a target for new anticoagulants. Hamostaseologie 2021;41:104-110.
- Piccini JP, Caso V, Connolly SJ, et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022;399:1383-1390.
- Akin I, Nienaber CA. Left atrial appendage occlusion: A better alternative to anticoagulation? World J Cardiol 2017;9:139-146.
- ClinicalTrials.gov. CHAMPION-AF Clinical trial. National Library of Medicine. Updated March 13, 2023. https://beta.clinicaltrials.gov/study/NCT04394546
- ClinicalTrials.gov. Amplatzer Amulet LAAO vs. NOAC (CATALYST). National Library of Medicine. Updated Feb. 27, 2023. https://beta.clinicaltrials.gov/study/NCT04226547
- Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol 2008;141:757-763.
- Montinari MR, Minelli S. From ancient leech to direct thrombin inhibitors and beyond: New from old. Biomed Pharmacother 2022;149:112878.
Nonvalvular atrial fibrillation is a highly prevalent cardiac arrhythmia in the United States and often can be complicated by a thromboembolic phenomenon, the most concerning of which is stroke. This article reviews the current evidence for the use of various anticoagulants, surgical techniques, and the left atrial appendage occlusion devices currently available for stroke prevention in atrial fibrillation.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
